According to Wikipedia, “any water used to optimize most-water based industrial processes, such as heating, cooling, processing, cleaning, and rinsing to that operational costs are reduced.” We who are involved within the industry of treatment of industrial water and removing dissolved mineral salts, hydrogen sulfide gas (H2S), and carbon dioxide gas (CO2) realize how important the proper treatment of water becomes in industrial applications for use in boiler feed systems and steam generation.
To comply with the tight operational standards and meet the industrial process requirements, water professionals must design and manufacture industrial water treatment equipment capable of performing decarbonation to remove CO2 to prevent carbonic acid formation. Decarbonation and Dearation Towers strip CO2 (carbon dioxide) and H2S (hydrogen sulfide) dissolved gases to prevent harmful scaling and corrosion and is a cost-effective water treatment solution.
 The Main Objective of Industrial Water Treatment
The Main Objective of Industrial Water Treatment
This prevents corrosion, scaling, and biological growth and ensures that water disposal standards are met. Without removing harmful mineral salts and corrosive gases, the industrial process equipment infrastructure, such as the piping, cooling towers, and boilers systems, would all suffer from the potential of corrosion, scaling, and ultimate system failure. A catastrophic system failure can occur when industrial water treatment processes become out of balance, and critical components are exposed to harmful corrosive scaling conditions. Essential functions such as steam production are often shut down due to scaling or corrosion that attacks boiler tube efficiency.
An industrial water treatment system typically uses several processes in series or parallel to meet the specified water quality for the industrial process, steam generation, or incorporation into a food or beverage. Several processes are commonly utilized within the industrial water treatment industry. Membrane filtration (reverse osmosis) (water filtering) will often be followed by deaeration, degasification, decarbonation, and additional ion exchange treatment. Chemical treatment, ozonation, and or U.V. ultraviolet are utilized for disinfection.
Membrane Filtration
More commonly known as “reverse osmosis,” it is a process where water is forced through a semipermeable membrane to remove molecules, ions, and larger particles. The membrane is encapsulated within a vessel or tube, and the applied pressure is used to overcome the osmotic pressure of the water. A high-pressure pump generates the pressure, and the pump pressure may vary depending on the type of membrane, the condition of the raw water, and the final water quality needed. The treated water is referred to as “permeate,” and the rejected water is referred to as “concentrate.” The solids of molecules, ions, or bacteria remain outside the membrane and are rejected through the end of the tube. Water with particulate or non-dissolved solids is measured by conductivity and is often reported as TSS (total suspended solids).
Duration
It is a process where steam and water are introduced into a deaerator “packed column tower” to force a “degasification” process of gases. Steam is utilized to heat water to near saturation while maintaining a lower-pressure vent. This is achieved by spraying water into a vertically packed deaerator tower equipment with a distribution system, which can be a weir tray or header lateral and multiple layers of trays or random packed media. Steam is typically introduced at the bottom of the tower and is forced to impact the falling water. The heated steam from the boiler feedwater system raises the water temperature to saturation level, releasing the dissolved gases from the water. Deaerators are primarily used in industrial applications to remove oxygen but can also remove CO2 (carbon dioxide gases).
Decarbonation and Degasification Towers
There are also vertical towers that utilize a distribution system and media bed, either PVC tray type or randomly packed media supported by a false bottom. A decarbonation and degasification tower uses a blower to create a cross-current airflow within the tower as it impacts the inlet feed water. Water is introduced at the top of the tower, and by gravity, it travels down and across the media bed as the cross-current airflow travels upwards and is exhausted at the top. Decarbonators and degasifiers can be either induced draft or forced draft design.
The efficiency of a decarbonator and degasifier in removing dissolved gases such as carbon dioxide (CO2) or hydrogen sulfide (H2S) is far greater than that of a deaerator as it does not require the introduction of steam. The decarbonator and degasifier efficiencies increase with water temperature rise, proper pH adjustment, media type selection, and airflow volume. The removal of the dissolved gases from the decarbonator and degasifier is based upon the chemistry law and is referred to as “Henry’s Law. Henry's law states that the amount of dissolved gas is proportional to its partial pressure in the gas phase. The proportionality factor is called the “Henry’s law constant.”
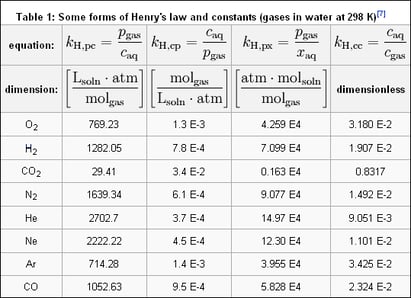
The gases are released when water passes through a decarbonator or degasification tower. The release of gases is possible by reshaping the water, exposing the gas molecules, and cross-current airflow, creating a disproportional pressure imbalance within the tower. Two types of processes can reshape water within a packed tower. The first process is called “controlled film,” which defines spreading the water thinly over a surface, allowing the molecules of dissolved gases to reach the surface of the water and be exposed to the cross-current airflow. The second process is called “impingement,” With impingement, water falls from one point to another where the water impacts and is “fractured,” causing molecules of dissolved gases to be exposed to the water's surface and be in contact with the cross current airflow. In both cases, the “steady state” of the water is altered by the cross-current airflow and the reshaping of the water to expose gas molecules. This creates the disproportional relationship of pressure and allows the gases to be stripped and ejected with the exhaust air stream. Existing water tension biding the dissolved gas molecules. Different media beds for decarbonation and degasification towers have different removal efficiencies. They are calculated by defining the values of NTU (number of turn units) or HTU (height of the turn unit). Media with higher NTU and HTU values typically yield higher removal efficiencies.
Ion Exchange Treatment
The industrial water treatment market protects critical components such as boiler feed systems and cooling towers. Without ion exchange, generating steam reliably and cost-effectively in many locations would be difficult, if not impossible. Ions are charged molecules or atoms; when an ionic substance is dissolved in water, the molecules dissociate into cations. “Cations” are positively charged particles. Negatively charged particles are referred to as “anions.” Ion exchange resins attract other molecules or atoms based on their electrical charge and replace them within the water process, leaving the removed molecule attached to the resin within the ion exchange system. Over time, the ion exchange system becomes saturated and must be back-washed, regenerated, and recharged. This is typically done with a backwash system and a regenerating solution near the equipment of the ionic exchange process.
Cationic exchangers are classified as strong (SAC) or weak (WAC) acid systems depending on the resin the unit is charged with. Strong and weak acid resins are utilized in the demineralization process at industrial water treatment locations. Strong acid cations are utilized for water softening, and weak acid cation systems are used for de-alkalization applications. Contaminants typically removed by cation resins include:
Calcium (Ca2+), Chromium (Cr3+ and Cr6+), Iron (Fe3+), Magnesium (Mg2+), Manganese (Mn2+), Radium (Ra2+), Sodium (Na+), and Strontium (Sr2+).
Depending on the resin the unit is charged with, anionic exchangers are typically classified as strong (SBA) or weak (WBA) base anion systems. Strong base anion resins are utilized in the demineralization process at industrial water treatment locations, while weak base anion systems are used for acid absorption. Contaminants typically removed by anion resins include:
Arsenic, Carbonates (CO3), Chlorides (Cl-), Cyanide (CN-), Fluoride, Nitrates (NO3), Perchlorate (ClO4-), Perfluoro octane sulfonate anion (PFOS), Perfluorooctanoic acid (PFOA), Silica (SiO2), Sulfates (SO4), and Uranium.
Industrial water disinfection is required to prevent the formation or contamination of bacteria in the water processes. Bacteria can damage equipment and products when it flourishes within an industrial water process, causing harmful and dangerous results when not properly prevented. Water processes involving the pharmaceutical, food, and beverage industries are meticulous regarding the treatment and disinfection of water. Chemicals like chlorine and ozonation are utilized as a means to kill and prevent the formation of bacteria. The disadvantage to using chemicals is the potential to alter the water chemistry further or impact the taste of the water. As an alternative, using ultraviolet light for disinfection has become widespread over the last 20 years. See the latest Water Consumption Statistics 2023.
The Need to Maintain Strict Water Quality Standards
The requirement to maintain good service and cleaning events of the water treatment systems involved in the industrial water processes. Decarbonation and degasification towers should be inspected and cleaned regularly, and the media beds should be cleaned and/or replaced to prevent poor performance and system failure. Without proper maintenance, these towers cannot efficiently remove hydrogen sulfide and carbon dioxide gases and remove or induce oxygen gases. Maintenance schedules control operational costs, which will rise when left unattended and can push an industrial operating budget into the red.
Industrial water treatment is also utilized to purify the water for other types of manufacturing where the water will come in direct contact with the manufactured products, like in the semi-conductor market, where the water is used to clean the surface during manufacturing. Removing harmful solids, minerals, iron, and mineral salts and preventing bacteria growth is a required standard. Industrial water can also be directly utilized and incorporated into the end products, such as the pharmaceutical or the food and beverage industry.
The Disposal of Industrial Water
The processes for treating the waste stream of an industrial application often mimic the fluent industrial water treatment process requirements. This is unsurprising because regulatory agencies have adopted “drinking water standards” for most reuse or wastewater disposal requirements and the wastewater disposal cost
in the USA and other countries. Water is treated through filtration, degasification, chemical injection for pH adjustment, and membrane filtration. Reverse osmosis is commonly used to return water to a “reuse” or safe to-discharge category.
In addition to treating industrial water, the air stream generated during the process must be treated because it can cause both human concern for workers and create a corrosive condition in the air, which can also damage the surface of surrounding equipment or instrumentation. Removing hydrogen sulfide gases is very corrosive and must often be treated with an industrial chemical or biological scrubber. Corrosive gases must be neutralized when treating industrial water in the semiconductor market or plating industry. A chemical scrubber utilizes a packed tower system with a distribution system at the top. The scrubber can be a single or double pass tower and will be equipped with randomly packed media. The type of scrubbing solution depends upon the type of gas that is being neutralized. For hydrogen sulfide scrubbers, chlorine and caustic are often deployed as effective methods to treat the hydrogen sulfide gas.
A more recent alternative that offers lower operating costs and is a more simplistic piece of equipment to operate and maintain is a biological scrubber. A biological odor control scrubber utilizes a packed tower design like a chemical scrubber. Still, instead of a recirculating reagent to oxidize or absorb the corrosive hydrogen sulfide gas, it uses a colony of bacteria cultured to digest the elements within the gas stream. The re-circulation process adds constant moisture to the media bed and injects nutrients when needed. Using a biological odor control scrubber over a chemical odor control or gas scrubber depends on the type of air stream, the concentration, and any fluctuation within the load rates. Unlike a chemical scrubber that can be charged with sufficient oxidation chemicals to instantly react to varying load rates, biological scrubbers do not adjust to high and low concentrations that change quickly.
Conclusion
Whether the industrial water process is used to convert to steam, clean, rinse, treat, or be incorporated into a final product, proper industrial water treatment and off-gas treatment remain the same. Design professionals have a wide selection of industrial water treatment equipment and systems produced by manufacturers like DeLoach Industries Inc. to assist them in developing the highest quality systems for each application.


 The Main Objective of Industrial Water Treatment
The Main Objective of Industrial Water Treatment






