Clean water is essential for sustaining life, and ensuring its purity is a priority for environmental agencies worldwide. However, a growing concern has emerged in recent years – the presence of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in drinking water. PFAS are persistent, human-made chemicals widely used in various industrial and consumer products, posing a significant threat to water sources and public health. Here, we'll explore the strategies and challenges involved in removing PFAS from drinking water, shedding light on the ongoing efforts by environmental agencies to manage and dispose of these persistent chemicals.
Understanding PFAS Contamination
PFAS contamination is a complex issue stemming from the widespread use of these chemicals in industries such as manufacturing, firefighting, and even everyday household products. These substances are known for their heat, water, and oil resistance, making them valuable for various applications. However, their persistence in the environment has raised serious concerns.
PFAS enter water sources through various pathways, including industrial discharges, wastewater treatment plants, and the use of firefighting foams. Once in the water, PFAS can accumulate over time, threatening aquatic ecosystems and, ultimately, human health. Long-term exposure to PFAS has been linked to adverse health effects, including developmental issues, immune system suppression, and an increased risk of certain cancers.
Strategies for Removing PFAS from Drinking Water
Addressing PFAS contamination requires a multifaceted approach, combining advanced water treatment technologies with comprehensive regulatory measures. Several strategies have been employed to remove PFAS from drinking water, each with its challenges.
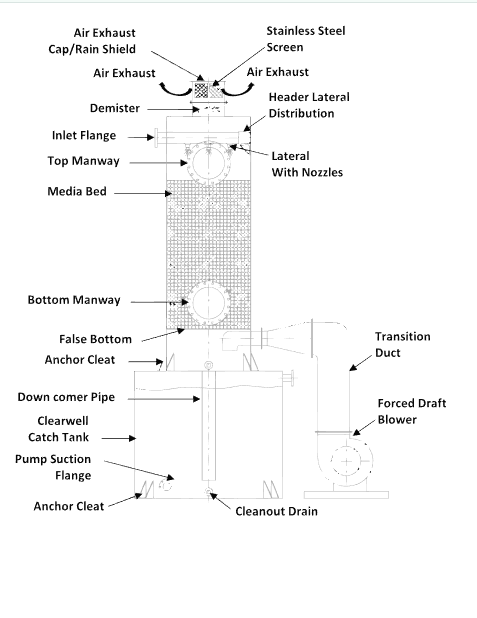
Activated Carbon Filtration
One widely adopted method for PFAS removal is activated carbon filtration. Activated carbon has a high affinity for PFAS compounds, effectively adsorbing them from the water. However, the efficiency of this method can be influenced by factors such as the type of activated carbon used, the water's chemical composition, and the presence of other contaminants. Additionally, the disposal of used activated carbon loaded with PFAS poses a significant challenge, as it can contribute to environmental contamination if not handled properly.
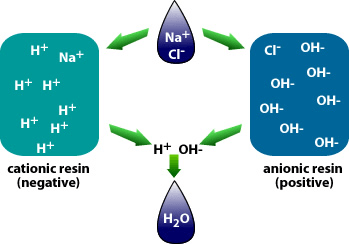
Ion Exchange Resins
Ion exchange resins are another technology employed to remove PFAS from drinking water. These resins replace PFAS ions with less harmful ions, effectively capturing and removing them from the water. While ion exchange can be highly effective, managing the used resins saturated with PFAS is challenging. Proper disposal methods must be implemented to prevent the release of these persistent chemicals back into the environment.
Advanced Oxidation Processes (AOPs)
Advanced Oxidation Processes (AOPs) involve using powerful oxidants to break down PFAS compounds into non-toxic byproducts. Techniques such as ozonation, ultraviolet (UV) irradiation, and hydrogen peroxide treatment fall under AOPs. While these methods show promise in PFAS degradation, they can be energy-intensive and may produce secondary pollutants. Balancing effectiveness with environmental impact remains a key challenge in implementing AOPs for large-scale water treatment.