Water turbidity refers to how transparent or translucent the water is when examining or testing it for any use.
Water turbidity can impact food and beverage, municipal, industrial, and aquaculture operations. Turbidity is caused by suspended or dissolved particles in the water that scatter light which causes the water to appear cloudy or even murky.
Different particles can cause turbidity, including sediments such as silts and clay, fine inorganic or organic matter, algae or soluble colored organic compounds, and microscopic organisms. Turbidity is measured in a value referred to as NTU, which means Nephelometric Turbidity Unit. The EPA requires a turbidity level no higher than 0.3 NTU in the USA, and if a member of the partnership of safe drinking water, then the level must not exceed 0.1 NTU.
High turbidity can create habitats for other harmful elements, such as bacteria or metals, that can accumulate onto the particles. This increases the health risk for a potable water system. In aquaculture operations, increased turbidity from silts and sediments can harm and harm marine life, so it must be removed to safe levels. For the food and beverage industry, the impact of high turbidity can be both a safety concern and a visual and noticeable quality concern because if the turbidity is high, it can alter the physical look of the final product, for example, a distillery.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
Safe drinking water,
De-Aeration,
decarbonator,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture,
Deagasification,
particulate matter,
filters,
Sand filters,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
turbidity
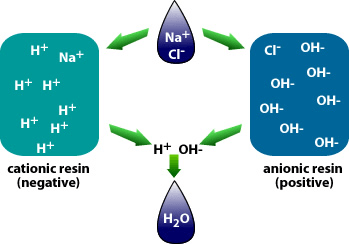
Water demineralization is also called deionization and is a process known as “Ion Exchange.”
In simple terms, water demineralization is “Water Purification.” The process involves removing dissolved ionic mineral solids from a feed-water process, typically for “Industrial” water applications. Still, it can also be utilized to remove dissolved solids from a water process for “Aquaculture,” “Food and Beverage,” and the “Municipal” markets.
Why is demineralization utilized? It can remove dissolved solids to near distilled water quality at a much lower capital and operational cost than other treatment processes such as membrane softening (Reverse Osmosis). Demineralization applies the science known as “Ion Exchange,” which attracts negative and positive charged ions and allows either to attach themselves to a negative ion depending on their respective current negative or positive charge during what is known as a resin cycle. In other technical articles, we will explore and go into more specific details on the science of the ion exchange process. Water that has dissolved salts and minerals has ions, either negatively charged ions known as “Anions” or positively charged ions known as “Cations.” To treat the water and remove these contaminants, the ions in the water are attracted to counter-ions, which have a negative charge. In a demineralization treatment process, there are pressure vessels that hold resin beads which are typically made of plastic. The beads are made from a plastic material with an ionic functional group that allows them to hold and maintain an electrostatic electrical charge. Some of these resin groups are negatively charged, referred to as “Anion” resins, while others hold a positive charge and are called “Cations” resins.
There are different applications to apply Ion exchange technologies, which is why you will often hear different terminology interchanged like deionization and demineralization. The raw water quality and the specific application will dictate the type of ion exchange process needed. For example, if the water contains a high level of hardness, the water will most likely contain Ca2+ or Mg2+ dissolved solids possessing a positive charge. To replace these hard ions, it is typical to utilize a resin bed with a salt ion like Na+. As the water passes over the resin bead material within the pressure vessel. The hard ions are replaced with the salt ion; therefore, all the hardness within the water is removed. However, the water will now contain a higher concentration of sodium ions, and this must be considered during the evaluation and selection process of the type of resin material to utilize for the specific application. If the water application requires high purity and the removal of as many solids as possible, then the term or process selected is referred to as demineralization.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
media packing,
Decarbonation,
ION Exchange Resin,
decarbonator,
degasifier,
RO system,
H2S Degasifier,
Aquaculture,
degassed water,
Co2 ph,
removal of CO2 from water,
Deagasification,
decarbonation of water,
hydrogen ion,
particulate matter,
municipal water systems,
industrial facilities,
automated control systems,
Ion exchange,
cations,
anions
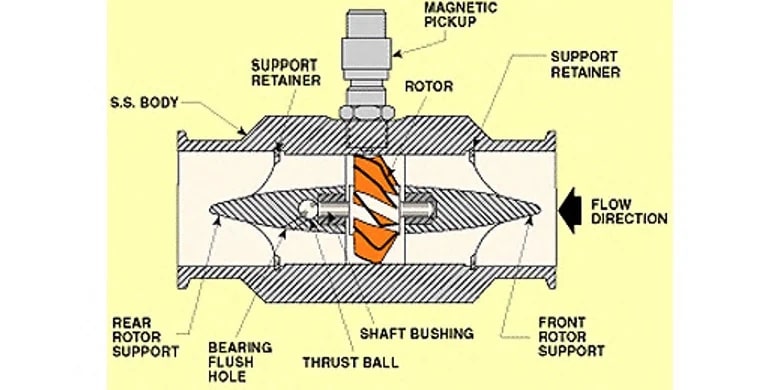
In water treatment systems it is often important to measure the rate at which water is flowing through the system. Data from flow measurement devices can be used to control chemical dosing, set pump speeds, control filter loading rates, inform maintenance programs, and other tasks necessary for the operation of a water treatment facility or on key components such as Degasification and Decarbonation systems or Biological Odor Control Systems. As with most types of instrumentation, there is an array of technologies that can be used for the task, each one with various strengths and optimal applications. For modern electronically controlled systems, the most common types of flow sensors used are axial turbine flowmeters, paddlewheel flowmeters, differential pressure/orifice plate flow transducers, and magnetic flowmeters. This article will briefly discuss the technology and features of each of these types.
A turbine flow meter,
consists of a tube that contains supports to hold a multi-bladed metal turbine in the center. The turbine is designed to have close clearance to the walls of the tubing such that nearly all of the water is made to flow through the turbine blades as it travels through the pipe. The turbine is supported on finely finished bearings so that the turbine will spin freely even under very low flows. As the turbine spins, a magnetic pickup located outside of the flowmeter housing is used to sense the tips of the turbine blade spinning past the pickup. An amplifier/transmitter is then used to amplify the pulses and either transmit them directly or convert the pulse frequency into an analog signal that is then sent to a programmable controller for further use elsewhere in the system. One advantage of a turbine flowmeter is that the electronics are separated from the fluid path. The magnetic pickup is the only electronic component, and it is installed outside of the turbine housing, reading the presence of the turbine blade tips through the wall of the sensor body. In clean water applications, this can be advantageous because the magnetic pickup can be replaced if needed without removing the turbine from service. However, the turbine itself covers most of the pipe area and creates back pressure in the system, requiring increased pumping energy to move a given amount of water. In Industrial Water Treatment or Filtration Treatment, turbines can also easily become fouled or jammed if they are used to measure water or other fluids with entrained solids, algae or bacteria cultures which cause significant accumulation, or corrosive chemical components that can degrade the turbine bearings.
Read More
Topics:
water quality,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
pumps,
Alkalinity,
Safe drinking water,
wastewater,
Recycling,
pharmaceutical water,
Aqua Farming,
Aquaculture,
Pipe Size,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
actuated valves,
pump controls,
Drinking Water,
Clean Water,
Water Test,
Water Test Kit,
DeLoach Industries,
civil engineers
The need to remove harmful water elements, such as Hydrogen Sulfide H2s and Carbon Dioxide CO2, from water in the pisciculture and aquaculture market is extremely important.
To achieve maximum results, the industry utilizes a treatment technology called “Degasification” and controls the pH precisely to maximize results. When utilizing equipment such as the DeLoach Industries degasification systems, the hydrogen sulfide, and carbon dioxide levels can be removed to 99.999% ug/l.
pH control with water degasification in water treatment is very important for aquaculture and the pisciculture market. In addition, there are a host of other organic and inorganic elements found in water, both naturally occurring and manmade, that require removal during some part of the water treatment process, and pH plays a significant role in the effectiveness of the treatment process.
Every application of degasification depends on pH adjustment to maximize results. As an example, the treatment of water may require the removal of hydrogen sulfide (H2S) to protect the species during the growth period. Hydrogen sulfide can be removed either as a “free” gas or requires the conversion of sulfides into (H2S) as a gas. You will often also see the need to adjust the pH of the water chemistry to maximize both the removal and the conversion to increase the efficiency of the equipment utilized to remove the hydrogen sulfide, such as a degasification tower or a degasifier.
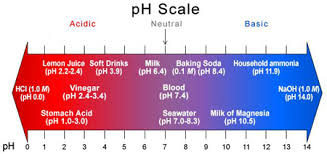
Why is pH important, and what it means in water?
Water pH is a term used to describe whether or not the water is “acidic” or “basic.” pH ranges in water can be from 0-14. 0 is the most acidic, and 14 is at the far end and is the most basic, leaving “7” as the neutral state. A pH of 7 is neither acidic nor basic. So, what causes pH to be acidic? In nature, the most common cause of a low acidic pH in water is carbon dioxide (CO2) which occurs naturally when photosynthesis, decomposition, or respiration occurs in nature. The increase in CO2 causes an increase in ions, producing a lower pH in a simplified explanation.
How does pH play such a significant role in Aquaculture and Pisciculture?
Removing certain harmful elements is typically required to safeguard the growth of most aquatic species, and elements such as sulfides, sulfates, and free H2S hydrogen sulfide gases are dangerous. They can often kill many types of aquatic life. To maximize the removal of hydrogen sulfide from water utilizing a DeLoach Industries degasification tower, it is important to maintain as close to a pH of 5 as possible. When the pH rises above 5, the ability to convert and strip the free H2S gas from the water diminishes. When a degasification tower operates within this specific range and if it has been designed with the higher efficient distribution systems such as the ones utilized by DeLoach Industries, removal efficiencies of 99.999%- 100% can be achieved. If the pH rises to 7 or above, the removal process becomes much more difficult, and typically you will have much lower results. The pH adjustment during the water treatment process is normally accomplished by adding commercially available acid, such as “Sulphuric Acid,” one of the most common within the municipal and food and beverage industry.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
The need for pH control with water degasification and decarbonation in water treatment includes almost every industry and includes;
The need for pH control with water degasification and decarbonation in water treatment includes almost every industry and including; Aquaculture, food, and beverage, industrial, municipal, and even pisciculture. In some water treatment applications, harmful gases such as Hydrogen Sulfide (H2S) are removed, while in other applications, Carbon Dioxide (CO2) or a combination of both. In addition, there's a host of other organic and inorganic elements found in water, both naturally occurring and manmade, that require removal during some part of the water treatment process.
In almost every application of degasification or decarbonation, you will hear or see the term pH used either by need or by the result. If, as an example, the water treatment application requires the removal of Hydrogen Sulfide (H2S) to be removed either as “free” gas or requires the conversion of Sulfides into (H2S) gas. You will often also see the need to adjust the pH of the water chemistry to maximize both the removal and the conversion to increase the efficiency of the equipment being utilized to remove the hydrogen sulfide, such as a degasification tower or commonly called a degasifier.
So, what is pH?
Water pH is a term used to describe whether or not the water is “acidic” or “basic.” pH ranges in water can be from 0-14. 0 is the most acidic, and 14 is at the far end and is the most basic, leaving “7” as the neutral state. A pH of 7 is neither acidic nor basic. So, what causes pH to be acidic? In nature, the most common cause of a low acidic pH in water is Carbon Dioxide (CO2) which occurs naturally when photosynthesis, decomposition, or respiration occurs in nature. The increase in CO2 causes an increase in ions, producing a lower pH in a simplified explanation.
How does pH play such a significant role in degasification and decarbonation?
As mentioned above in the example of the removal of certain harmful elements such as sulfides, sulfates, and free H2S hydrogen sulfide gases, to maximize the removal from water utilizing a degasification tower, it is essential to maintain as close to a pH of 5 as possible. When the pH rises above 5, the ability to convert and strip the free H2S gas from the water diminishes. When a degasification tower operates within this specific range and if it has been designed with the higher efficient distribution systems such as the ones utilized by DeLoach Industries, removal efficiencies of 99.999%- 100% can be achieved. If the pH rises to seven or above, the removal process becomes much more complex, and typically, you will have much lower results. The pH adjustment during the water treatment process is typically accomplished by adding commercially available acid, such as “sulphuric acid,” one of the most common in the municipal and food and beverage industry.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
odor control,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
Chemical Odor,
pH levels,
Decarbonation,
dissolved gases,
carbon dioxide,
degasifier,
gases,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture
Optimizing Water Quality and Enhancing Efficiency.
To enhance and balance the water quality in aquaculture and pisciculture operations, the industry is recognizing the benefits of utilizing a type of water treatment called “Forced Draft Degasification” to remove CO2 (Carbon Dioxide) and H2S (Hydrogen Sulfide) gases and oxidize other elements such as Iron or Magnesium.
Removing these elements from the water process improves the quality of the water and aids in the balancing of the pH without the need for additional chemicals. This also reduces the risk of unnecessary and preventable loss of life to your aqua farming project. Keeping the ph at the proper level will enable a good healthy environment and will prevent further problems that may occur due to the ph is not kept in a stable state.
Read More
Topics:
De-Aeration,
carbon dioxide,
decarbonator,
degasifier,
gases,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
To enhance and control the production and quality of seafood grown and harvested.
The industry increasingly focuses on constructing in-house aquaculture fish farms, commonly called aqua farming. The most popular species of aqua farming continue to be salmon, tilapia, catfish, and carp. Increased interest in the United States has developed aqua farming facilities in southern Florida with favorable climate and water conditions.
When considering several types of fish species to grow for harvest, it is important to remember the need to control the water quality. If the aqua farm is intended to utilize man-made tanks, they will depend upon a constant flow of incoming water. If the aqua farm focuses on salmon, the water quality and temperature play a major role in the operation's mortality rates and production yields.
Having water with too high of hydrogen sulfide, carbon dioxide, total Organic carbons, and even turbidity can increase mortality rates among the younger fish species and is especially critical to salmon.
Having high levels of metals
Such as Iron that is identified as either “ferric” (Fe-) or “ferrous” (FE+2) and is naturally occurring within the Florida waters and other parts of the US will cause significant damage to young salmon species because the metal accumulates within the gills of the fish causing suffocation. Other metals are also detrimental to fish, including copper, aluminum, arsenic, cadmium, chromium, Lead, manganese, and mercury, to name a few, and the water quality must be evaluated and tested in the early stages of design to anticipate the required types of process systems needed.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
Decarbonation,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
carbonic acid,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
Water Treatment
When planning and designing a man made on land aquaculture or pisciculture facility.
The most important key element is the quality of the water. For operations developing in Florida or the Caribbean it is important to remember that water quality varies in Florida and other states in the US and typically requires some type of water treatment. For fresh and salt water land based farms that utilize tanks located inside of a building the water needs to be treated and pure from any naturally occurring contaminants such as hydrogen sulfide (H2S), iron (Fe+), and even carbon dioxide (CO2).
The most cost effective way to treat incoming water for aquaculture farming and remove hydrogen sulfide, iron, and lower carbon dioxide is the use of a “degasification” tower. A degasification tower or degasifier is a piece of process equipment. Degasifiers can also be referred to as a “decarbonator” or “air stripper” or even “aeration tower”. The degasification tower is a vertical column designed to remove certain types of contaminants by “stripping” the molecules of converted gases and expelling them from the water as a gas. The science is based upon “Henry’s Law” and it relies upon the disproportionate varying vapor pressures of gases.
If the incoming raw water contains levels of sulfides or hydrogen sulfide gases it is recommended to remove the hydrogen sulfide to improve the water quality and reduce the risk of the development and formation of bacteria that can thrive on the Sulfur. In addition hydrogen sulfide is corrosive and will cause harm to other components within the process if left untreated. It is important to adjust the pH of the raw feed water prior to degasification to ensure full conversion of the sulfides into hydrogen sulfide gas (H2S) to enable the degasification process to perform and remove up to 99.99% of the harmful contaminants without adding additional chemicals. This saves money and improves quality of the product!
Read More
Topics:
water quality,
degasification,
pH levels of water,
water treatment,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
Decarbonation,
Caribbean,
carbon dioxide,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
Read More
Topics:
water treatment issues,
water quality,
pH levels of water,
aeration,
water treatment,
advanced treatment solutions,
fiberglass,
About DeLoach Industries,
fabrication,
biological scrubber,
Chemical Odor,
media packing,
pH levels,
Decarbonation,
De-Aeration,
decarbonator,
boiler system,
distillation,
degasifier,
RO system,
H2S Degasifier,
Fish Farming,
Aquaculture,
Pisciculture,
Biological Odor Control Scrubber,
Biological odor control,
removal of CO2 from water,
Deagasification,
decarbonation of water,
Sand filters,
Filter Media,
municipal water systems,
greensand,
DeLoach Industries, Inc.,
Drinking Water