PFOA and PFOS are man-made chemicals used in various products to simplify life.
Forever chemicals, also known as synthetic chemicals called PFAS, have gained recognition. Scientists created these chemicals to make products resistant to water, stains, and sticking. The United States initially utilized them in the 1950s.
DuPont introduced Teflon in the 1950s to help Americans have nonstick cookware and make their lives easier. Americans and people from other countries liked this new improvement and soon used these substances in many different products.
These chemicals are resistant to water and lipids, so they don't break down and last a long time in the environment.
Over time, companies have used these chemicals in manufacturing various products, such as firefighting foam, food packaging, and cosmetics. As a result, these chemicals have entered the air, water, soil, and food production. They discontinued the use of PFAS and their other compounds in the mid-1970s.
People believe that contamination has affected more than 7000 metric tons of Fluorochemicals. PFOAs and PFOS, which can cause various health problems, have exposed many Americans and people in the USA.
PFOA chemicals contaminated 1% of public drinking water supply systems in 2016. The EPA did not regulate safe levels of PFOA and PFOS in drinking water systems for many years.
Read More
Topics:
water quality,
advanced treatment solutions,
pH levels,
Safe drinking water,
RO system,
particulate matter,
Filter Media,
municipal water systems,
DeLoach Industries, Inc.,
Drinking Water,
Clean Water,
PFA's,
DeLoach Industries,
nylon,
Cosmetics,
reverse osmosis,
water process system,
removing PFAS & PFOS,
pfas exposure,
health effects of pfas,
exposure to pfas,
nonstick cookware,
food packaging,
water treatment standards,
PFOS,
safe drinking water act,
pfoa regulations,
the environmental protection agency,
drinking water standards,
water resistant clothing,
environmental safety,
forever chemicals
If you’ve been reading the news lately, you know nanoparticles are not so great. In everything from cosmetics to water filters, nanoparticles have been shown to cause various health problems. But what exactly are nanoparticles, and how can you protect yourself from their harmful effects? Let’s answer these questions and more with this quick guide on removing nanoparticles from your drinking water.
Read More
Topics:
water treatment issues,
water quality,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
safety,
Safe drinking water,
Global,
distillation,
RO membrane,
RO system,
particulate matter,
filters,
municipal water systems,
residential well water systems,
DeLoach Industries, Inc.,
Drinking Water,
Clean Water,
Water Test,
Water Test Kit,
DeLoach Industries,
technology,
minerals,
temperature,
nanoparticles,
Cosmetics,
Nano,
make-up,
organ function,
contaminants,
pressure filters,
reverse osmosis,
carbon filters,
UV filters,
activated carbon
Water turbidity refers to how transparent or translucent the water is when examining or testing it for any use.
Water turbidity can impact food and beverage, municipal, industrial, and aquaculture operations. Turbidity is caused by suspended or dissolved particles in the water that scatter light which causes the water to appear cloudy or even murky.
Different particles can cause turbidity, including sediments such as silts and clay, fine inorganic or organic matter, algae or soluble colored organic compounds, and microscopic organisms. Turbidity is measured in a value referred to as NTU, which means Nephelometric Turbidity Unit. The EPA requires a turbidity level no higher than 0.3 NTU in the USA, and if a member of the partnership of safe drinking water, then the level must not exceed 0.1 NTU.
High turbidity can create habitats for other harmful elements, such as bacteria or metals, that can accumulate onto the particles. This increases the health risk for a potable water system. In aquaculture operations, increased turbidity from silts and sediments can harm and harm marine life, so it must be removed to safe levels. For the food and beverage industry, the impact of high turbidity can be both a safety concern and a visual and noticeable quality concern because if the turbidity is high, it can alter the physical look of the final product, for example, a distillery.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
Safe drinking water,
De-Aeration,
decarbonator,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture,
Deagasification,
particulate matter,
filters,
Sand filters,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
turbidity
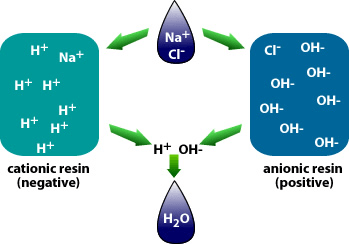
Water demineralization is also called deionization and is a process known as “Ion Exchange.”
In simple terms, water demineralization is “Water Purification.” The process involves removing dissolved ionic mineral solids from a feed-water process, typically for “Industrial” water applications. Still, it can also be utilized to remove dissolved solids from a water process for “Aquaculture,” “Food and Beverage,” and the “Municipal” markets.
Why is demineralization utilized? It can remove dissolved solids to near distilled water quality at a much lower capital and operational cost than other treatment processes such as membrane softening (Reverse Osmosis). Demineralization applies the science known as “Ion Exchange,” which attracts negative and positive charged ions and allows either to attach themselves to a negative ion depending on their respective current negative or positive charge during what is known as a resin cycle. In other technical articles, we will explore and go into more specific details on the science of the ion exchange process. Water that has dissolved salts and minerals has ions, either negatively charged ions known as “Anions” or positively charged ions known as “Cations.” To treat the water and remove these contaminants, the ions in the water are attracted to counter-ions, which have a negative charge. In a demineralization treatment process, there are pressure vessels that hold resin beads which are typically made of plastic. The beads are made from a plastic material with an ionic functional group that allows them to hold and maintain an electrostatic electrical charge. Some of these resin groups are negatively charged, referred to as “Anion” resins, while others hold a positive charge and are called “Cations” resins.
There are different applications to apply Ion exchange technologies, which is why you will often hear different terminology interchanged like deionization and demineralization. The raw water quality and the specific application will dictate the type of ion exchange process needed. For example, if the water contains a high level of hardness, the water will most likely contain Ca2+ or Mg2+ dissolved solids possessing a positive charge. To replace these hard ions, it is typical to utilize a resin bed with a salt ion like Na+. As the water passes over the resin bead material within the pressure vessel. The hard ions are replaced with the salt ion; therefore, all the hardness within the water is removed. However, the water will now contain a higher concentration of sodium ions, and this must be considered during the evaluation and selection process of the type of resin material to utilize for the specific application. If the water application requires high purity and the removal of as many solids as possible, then the term or process selected is referred to as demineralization.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
media packing,
Decarbonation,
ION Exchange Resin,
decarbonator,
degasifier,
RO system,
H2S Degasifier,
Aquaculture,
degassed water,
Co2 ph,
removal of CO2 from water,
Deagasification,
decarbonation of water,
hydrogen ion,
particulate matter,
municipal water systems,
industrial facilities,
automated control systems,
Ion exchange,
cations,
anions
In water treatment, it is often required to remove small particulate matter from the raw water.
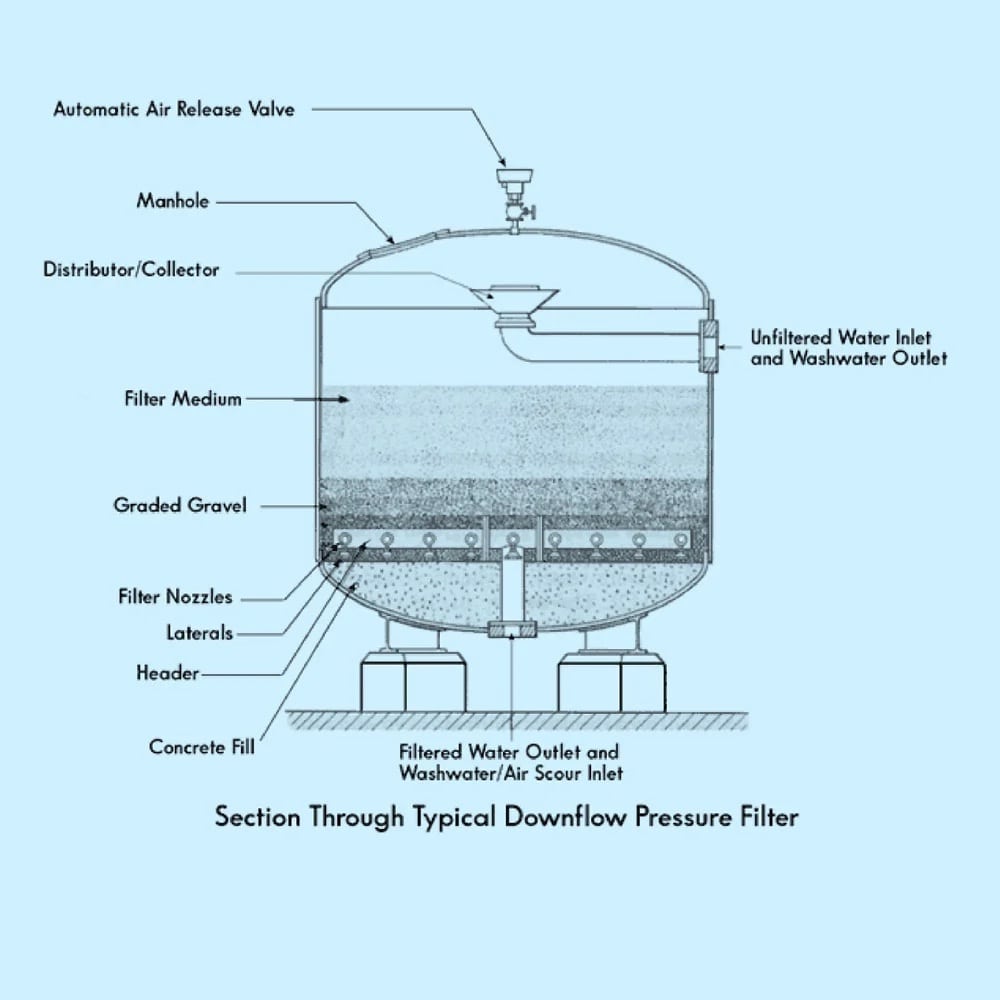
One of the most cost-effective ways to accomplish this is with a pressure filter. Sometimes referred to as “sand filters,” a pressure filter consists of a rigid filter vessel capable of withstanding internal pressure, combined with pipework to distribute and collect water and one or multiple types of filter media. Pressure filters are commonly used in municipal water systems, industrial facilities, residential well water systems, and swimming pools. Typical pressure filter construction is shown below:
At the top of the filter vessel, a distributor is used to break up and distribute the water flow so that there are no concentrated flow jets that stir up the media bed. Inflow distributors are usually oriented to direct flow at the top of the vessel to disperse the flow further. Below the distributor is the primary filter bed. The filter bed contains fine-grained media, most often sand, including crushed anthracite coal, activated charcoal, garnet, or other granular bulk products. The media bed is the thickest layer in the filter vessel and is the region that does the actual filtering of the water or other fluid. Below the media bed will be one or more support layers. These will usually be larger-sized gravel that is chosen to support the filter bed while allowing high flow through the support layer and into the outflow header. The outflow header can take several forms but is often composed of a large central pipe with multiple smaller pipes or “laterals” attached. The laterals are slotted or perforated. This allows the pressurized water to flow into the laterals and out through the outflow header into the downstream components of the water treatment system.
Read More
Topics:
particulate matter,
filters,
Pressure filter,
Sand filters,
Filter Media,
municipal water systems,
industrial facilities,
residential well water systems,
greensand,
DeLoach Industries, Inc.,
backwash,
automated control systems,
actuated valves,
pump controls