Water purification is critical in industrial operations, especially when it comes to removing dissolved gases that can cause corrosion and compromise the efficiency of equipment. Various methods exist to address these issues, with Forced Draft Degasification (FDD) systems often cited as one of the best options. However, while FDD systems have clear advantages, they may not always be the ideal solution depending on specific needs. In this article, we'll explore the ins and outs of Forced Draft Degasification, weigh its benefits and drawbacks, and compare it to alternative methods to help you determine if it's truly the best choice for your water purification needs.
Understanding Forced Draft Degasification Systems
Forced Draft Degasification systems are designed to remove dissolved gases, such as carbon dioxide (CO2) and hydrogen sulfide (H2S), from water, which can cause significant problems in industrial equipment. The principle behind FDD is simple but effective: water flows through a vertically structured tower, typically packed with media that maximizes the surface area. As water travels downward, a cross-current of air is forced through the system, helping to strip out unwanted gases.
This interaction between air and water allows for the efficient removal of these gases, which otherwise would contribute to the deterioration of industrial equipment through corrosion. Corrosive environments not only shorten the lifespan of machinery but also increase maintenance costs, leading to inefficiencies that can disrupt entire operations. The goal of FDD systems is to mitigate these risks, making water safe for industrial use and protecting expensive equipment investments.
These systems are widely used in industries such as power generation, chemical processing, and oil refining, where the presence of dissolved gases can lead to substantial equipment failure or process inefficiencies. The vertical design of the FDD system enables it to handle large volumes of water efficiently, making it ideal for high-capacity industrial needs. The simplicity of the FDD system, combined with its ability to consistently remove dissolved gases, makes it a preferred choice for many industrial operations that prioritize reliability and cost-effectiveness in their water treatment systems.
Read More
Topics:
hydrogen sulfide (H2S),
carbon dioxide,
degasifier,
Deagasification,
DeLoach Industries, Inc.,
Forced Draft,
Industrial water treatment
In modern industrial water treatment, advancements in technology and processes have revolutionized the way contaminants are removed from water.
This blog explores the integration of NSF/ANSI 61 certified systems, artificial intelligence in water treatment, and cutting-edge processes such as decarbonation and degasification. We'll also discuss the key differences between forced draft and induced draft degasification towers, helping you make informed decisions while designing your Industrial Water Treatment System.
-
NSF/ANSI 61-Certified Water Treatment Systems: To ensure the safety and quality of water treatment equipment, NSF/ANSI 61 certification has become a crucial standard. This certification verifies that materials and components used in water treatment systems comply with health and safety requirements. When selecting a water treatment solution, opting for NSF/ANSI 61 certified systems guarantees peace of mind and adherence to the highest industry standards.
-
Harnessing Artificial Intelligence in Water Treatment: Artificial intelligence (AI) has penetrated various industries, and water treatment is no exception. Integrating AI into water treatment processes allows for more efficient and optimized operations. AI-driven systems can monitor water quality in real-time, predict system failures, optimize chemical dosing, and reduce energy consumption. By leveraging AI technologies, water treatment facilities can enhance their overall performance and streamline resource utilization.
-
Decarbonation and Degasification Systems: Decarbonation and degasification are essential processes in industrial water treatment, particularly in pH levels in water and the ability to control removing the contaminants. These processes target the removal of carbon dioxide (CO2) and other dissolved gases from water to improve its quality. Two key systems used for this purpose are the decarbonator and aeration system.
Read More
Topics:
degasification,
advanced treatment solutions,
biological scrubber,
NSF/ANSI 61,
Chemical Odor,
Decarbonation,
Safe drinking water,
De-Aeration,
decarbonator,
degasifier,
degassed water,
ansi61,
nsf/ansi61,
Deagasification,
decarbonation of water,
DeLoach Industries, Inc.,
Drinking Water,
Industrial Odor Control,
DeLoach Industries,
contaminants,
process system,
safe drinking water act,
drinking water standards,
environmental safety,
air emissions,
Forced Draft,
Induced Draft
Water turbidity refers to how transparent or translucent the water is when examining or testing it for any use.
Water turbidity can impact food and beverage, municipal, industrial, and aquaculture operations. Turbidity is caused by suspended or dissolved particles in the water that scatter light which causes the water to appear cloudy or even murky.
Different particles can cause turbidity, including sediments such as silts and clay, fine inorganic or organic matter, algae or soluble colored organic compounds, and microscopic organisms. Turbidity is measured in a value referred to as NTU, which means Nephelometric Turbidity Unit. The EPA requires a turbidity level no higher than 0.3 NTU in the USA, and if a member of the partnership of safe drinking water, then the level must not exceed 0.1 NTU.
High turbidity can create habitats for other harmful elements, such as bacteria or metals, that can accumulate onto the particles. This increases the health risk for a potable water system. In aquaculture operations, increased turbidity from silts and sediments can harm and harm marine life, so it must be removed to safe levels. For the food and beverage industry, the impact of high turbidity can be both a safety concern and a visual and noticeable quality concern because if the turbidity is high, it can alter the physical look of the final product, for example, a distillery.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
Safe drinking water,
De-Aeration,
decarbonator,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture,
Deagasification,
particulate matter,
filters,
Sand filters,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
turbidity
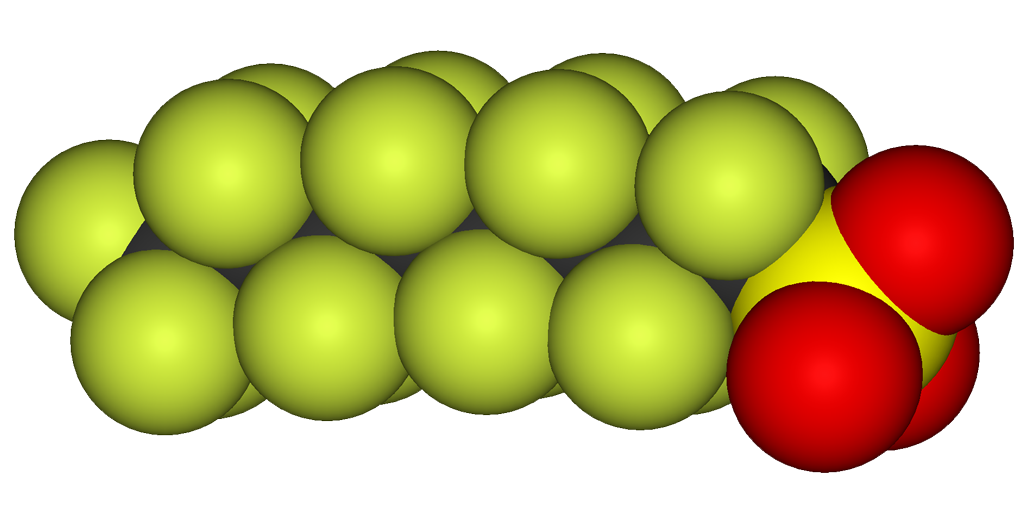
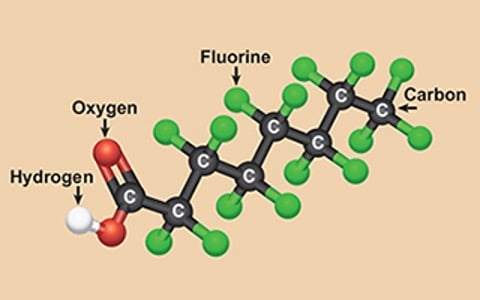
The EPA and other world health organizations have sounded the alarm on the dangers and health impacts of being exposed to per- and polyfluoroalkyl substances (PFASs & PFOAs) also known as the forever chemicals.
In response, federal and state regulators are adopting new water quality guidelines and laws to address these contaminants in our drinking water systems and groundwater pollution. It's a pervasive issue, as PFASs can be found in various types and over 4,700 different variations, each with at least three polyfluorinated carbon atoms.
With more than 10,000 types of PFASs introduced into products, it's no wonder that the quality of drinking water in the USA and other countries has been compromised. But what exactly are PFASs? These are fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom. While they do not contain atoms like hydrogen, chlorine, bromine, or iodine, any chemical with a perfluorinated (CF3) or perfluorinated (CF2) component falls under the PFAS category. However, there are a few exceptions.
PFASs can be further classified into subgroups such as surfactants, perfluorosulfonic acids, perfluorooctane sulfonic acids, perfluorocarboxylic acids, and perfluorooctanoic acids (commonly referred to as PFOSs and PFOAs). These persistent organic pollutants, also known as "forever chemicals," pose a significant challenge due to their resistance to environmental degradation. As a result, they are found in humans, animals, and water supplies across the USA.
Read More
Topics:
degasification,
NSF/ANSI 61,
Decarbonation,
Safe drinking water,
ansi61,
Co2 ph,
CO2 in water,
Deagasification,
hydrogen ion,
DeLoach Industries, Inc.
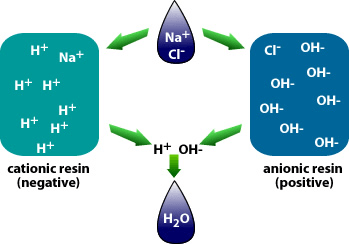
Water demineralization is also called deionization and is a process known as “Ion Exchange.”
In simple terms, water demineralization is “Water Purification.” The process involves removing dissolved ionic mineral solids from a feed-water process, typically for “Industrial” water applications. Still, it can also be utilized to remove dissolved solids from a water process for “Aquaculture,” “Food and Beverage,” and the “Municipal” markets.
Why is demineralization utilized? It can remove dissolved solids to near distilled water quality at a much lower capital and operational cost than other treatment processes such as membrane softening (Reverse Osmosis). Demineralization applies the science known as “Ion Exchange,” which attracts negative and positive charged ions and allows either to attach themselves to a negative ion depending on their respective current negative or positive charge during what is known as a resin cycle. In other technical articles, we will explore and go into more specific details on the science of the ion exchange process. Water that has dissolved salts and minerals has ions, either negatively charged ions known as “Anions” or positively charged ions known as “Cations.” To treat the water and remove these contaminants, the ions in the water are attracted to counter-ions, which have a negative charge. In a demineralization treatment process, there are pressure vessels that hold resin beads which are typically made of plastic. The beads are made from a plastic material with an ionic functional group that allows them to hold and maintain an electrostatic electrical charge. Some of these resin groups are negatively charged, referred to as “Anion” resins, while others hold a positive charge and are called “Cations” resins.
There are different applications to apply Ion exchange technologies, which is why you will often hear different terminology interchanged like deionization and demineralization. The raw water quality and the specific application will dictate the type of ion exchange process needed. For example, if the water contains a high level of hardness, the water will most likely contain Ca2+ or Mg2+ dissolved solids possessing a positive charge. To replace these hard ions, it is typical to utilize a resin bed with a salt ion like Na+. As the water passes over the resin bead material within the pressure vessel. The hard ions are replaced with the salt ion; therefore, all the hardness within the water is removed. However, the water will now contain a higher concentration of sodium ions, and this must be considered during the evaluation and selection process of the type of resin material to utilize for the specific application. If the water application requires high purity and the removal of as many solids as possible, then the term or process selected is referred to as demineralization.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
media packing,
Decarbonation,
ION Exchange Resin,
decarbonator,
degasifier,
RO system,
H2S Degasifier,
Aquaculture,
degassed water,
Co2 ph,
removal of CO2 from water,
Deagasification,
decarbonation of water,
hydrogen ion,
particulate matter,
municipal water systems,
industrial facilities,
automated control systems,
Ion exchange,
cations,
anions
Per- and polyfluorinated substances (PFAS), known as "forever chemicals," have long been utilized in various consumer products due to their exceptional properties.
However, the challenge lies in effectively treating or eliminating PFAS once they enter the environment or water supply. This blog will focus on the technological advancements in removing PFAS and perfluorooctanoic acids (PFOAs) from water sources. By exploring different treatment methods, such as activated carbon absorption, ion exchange resins, and reverse osmosis, and simply avoiding PFOA and PFOS, we can better understand the available options for mitigating these persistent chemicals in water.
Activated Carbon Absorption
One of the earliest technologies employed for PFAS removal is activated carbon absorption. This method involves the use of specially treated carbon materials that effectively adsorb PFAS compounds from water sources. The activated carbon's large surface area and porous structure allow it to trap and retain PFAS molecules. This technology has proven effective in removing PFAS, including PFOAs, from drinking water and environmental sources. However, periodic treatment and regeneration of the activated carbon are necessary to maintain its efficacy.
Read More
Topics:
degasification,
iron oxidation,
water treatment,
advanced treatment solutions,
water plant,
ION Exchange Resin,
Safe drinking water,
wastewater,
degasifier,
RO system,
Deagasification,
PFA's,
technology,
contaminants,
reverse osmosis,
carbon filters,
activated carbon,
removing PFAS & PFOS,
pfas exposure,
health effects of pfas,
nonstick cookware,
wastewater treatment systems,
PFOS,
pfoa regulations,
drinking water standards,
water resistant clothing,
environmental safety
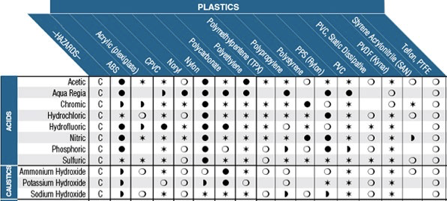
In process control systems, it is often required to handle fluids that have a harsh chemical nature. In these cases, it is necessary to be aware of material-chemical compatibility. Chemical compatibility is a general term referring to the way a specific chemical interacts with a specific material. This information is taken into consideration when selecting materials for construction for tanks, valves, pipework, tubing, and other devices that may encounter harsh chemicals. Common chemical types that are used in process systems are acids, bases, corrosives and oxidizers, and hydrocarbons. Typical chemical-resistant materials include natural and synthetic rubbers, vinyl polymers, fluoropolymers, and stainless steel. In order to determine which materials are compatible with certain chemicals, a chemical compatibility chart is often used. A chemical compatibility chart contains tabulated data about how a given material interacts with a given chemical.
Often, the manufacturer of the equipment or material in question will have their own compatibility chart for their specific goods. Most compatibility charts will have the same type of information. Materials will be categorized along one axis of the table, with fluids or gasses categorized along the other axis. At the intersection of a material with a fluid, you will find an indication of the level of compatibility. Some charts will use an A-F categorization, others may use a more graphical style. Most charts will be accompanied by a key or guide that explains how to use the table. There may also be multiple concentration levels and temperature ranges for a given fluid in cases where the distinction makes a difference with compatibility.
Read More
Topics:
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
caustic,
Decarbonation,
decarbonator,
degasifier,
Deagasification
When do you know if your decarbonation system needs service?
When a degasification tower or decarbonator becomes fouled, several indicators identify you may have a problem or that it's time to clean your system. If the efficiency of the degasifier has dropped, you will see an increased consumption rate of chemicals. If you remove less hydrogen sulfide gas from the degasifier, chlorine consumption will increase. When you increase the amount of chemical reaction occurring in the water, you will see an increase in the TSS levels and a drop in water quality. As the H2S reacts with chlorine, more solids will form and be present in the water, and the water quality will diminish.
Another indicator of a fouling condition is the pH adjustment in the Industrial Water Treatment industry. You are required to meet the set standards. As the performance of the tower drops, the removal of CO2 will also drop, leaving a higher pH level than may be desired. A quick inspection to check out the media bed should be performed. Also, do not forget to inspect the distribution system at the top of your tower and remember that all distribution systems are not alike, and inspecting the condition of each of them may require additional effort on your part. With a header lateral system, you need to inspect the distribution nozzles, but with a Weir or Tray type, you will need to check the amount of scale or fouling building up on the Weir edge or in the bottom of the pan. If the Weir edge becomes fouled unevenly, it will create "Channeling" of the water and increase the initial hydraulic load to a concentrated point on the media bed.
Read More
Topics:
water treatment issues,
blower maintenance,
aeration,
water treatment,
advanced treatment solutions,
degasifier,
Deagasification,
decarbonation of water
Ammonia (AM) is a common water pollutant that significantly impacts the water process industry.
It is not just polluting water bodies but also aqua wells and humidifiers. Generally, AM is produced from human sweat and urine and created from synthetic ammonia in industrial processes.
Ammonia has three types of amines – primary, secondary, and tertiary – all are toxic for humans and aquatic life.
- Primary Amine has two carbon and one nitrogen atom, also called methylamine or CHNH2.
- Secondary Amine has two nitrogen atoms with no carbon atom between them, also called Dimethylamine or CH2(NH)CH3.
- Tertiary Amine has three nitrogen atoms with no carbon atoms between them; thus, it’s called Trimethylamine or CH3C(NH)CH3.
In natural conditions, primary Amide bacteria produce Amide under high-temperature conditions. In an aqueous solution and soil environments with high pH levels (>6).
Primary amide can form by the dehydrogenation of nitriles, such as acetonitrile, which are further oxidized to form acetic acid.
Primary amide form by alkaline hydrolysis of nitro compounds such as 2-nitrophenol.
Process systems often need to recognize when the Degasification or Decarbonation system is failing or underperforming.
Read More
Topics:
Decarbonation,
decarbonator,
degasifier,
Amine,
Ammonia,
Deagasification,
Filter Media,
distribution system,
blower motor,
process system,
frequent inspections
Water treatment towers and storage tanks are high places that require special precautions when entering. While the majority of people who enter these locations for work can be trusted, there are some hazards that make it more important than usual to follow safety procedures.
These locations can get very hot and humid, and can also be filled with harmful chemicals and microorganisms that can cause serious health issues if inhaled or absorbed through the skin. Therefore, the general standard for workplace safety is much higher when entering locations like these.
Make sure you have read and understood the following information about safety when entering a water treatment plant. It will help you understand how to stay safe and protect yourself from harm when entering a water treatment plant. normal installation, maintenance, or even emergency repairs, it is often required to enter into a water treatment tower (degasifier, air stripper, decarbonator, or clear well/ storage tank). When this occurs, full safety protocols should be followed at all times, in accordance with OSHA regulations. A tower or tank B classification is a "Confined Space" location. For more information visit the OSHA confined space regulations page.
In addition, there are other safety risks that an operator or technician can be exposed to while inside these types of closed locations. The risk can come from fumes of hydrogen sulfide (H2S), chlorine from an injection line, or a lack of oxygen O2. A proper confined space permit should be prepared and only technicians with proper training and certifications should enter into these types of confined spaces.
Read More
Topics:
water treatment issues,
water quality,
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
safety,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
media packing,
scaling,
caustic,
Safe drinking water,
dissolved gases,
wastewater,
carbon dioxide,
degasifier,
gases,
Ammonia,
what is a scrubber,
Hydrogen Sulfide formula,
Deagasification,
Filter Media,
DeLoach Industries, Inc.,
Drinking Water,
Clean Water,
Contaminated Water,
OSHA