The Fundamentals of Water Decarbonation
Water decarbonation, also known as degasification, is the process of removing carbon dioxide (CO2) from water. This procedure is essential in various sectors, including aquaculture, municipal water treatment, industrial processes, and the food & beverage industry. The presence of CO2 in water can lead to multiple problems, including equipment corrosion, reduced efficiency in water treatment systems, and negative impacts on aquatic life.
Read More
Topics:
water treatment issues,
degasification,
water treatment,
Decarbonation,
degasifier,
removal of CO2 from water,
CO2 in water,
decarbonation of water,
DeLoach Industries, Inc.,
Henry's Law
I will explore the potential risks of exposure to two members of a family of man-made chemicals called PFAS.
These chemicals are PFOA and PFOS, "poly-fluoroalkyl substances."
I will discuss the sources of PFOA and PFOS. These include leaching from industrial sites, the use of consumer products, and food and water contamination.
I will also discuss the exposure pathways of PFOA and PFOS. I will examine the regulations and guidelines for the use of these chemicals. I will also investigate their impact on the environment and various industries.
I will guide long-term human health effects.
This guide covers the potential risks of pfo's and pfoa's. It explains their sources and exposure pathways. It also looks at regulations and guidelines for their usage and impact on the environment and industries.

Introduction to PFOA and PFOS
Read More
Topics:
water treatment issues,
water quality,
water treatment,
advanced treatment solutions,
FDA,
Safe drinking water,
wastewater,
Global,
RO system,
DeLoach Industries, Inc.,
Drinking Water,
PFA's,
DeLoach Industries,
Cosmetics,
make-up,
water process system,
removing PFAS & PFOS,
pfas exposure,
health effects of pfas,
nonstick cookware,
wastewater treatment system,
water treatment standards,
PFOS,
safe drinking water act,
pfoa regulations,
the environmental protection agency,
drinking water standards,
adverse health effects,
water resistant clothing,
environmental safety
If you’ve been following the news, you know a growing problem with PFAS (per- and poly-fluoroalkyl substances) exists.
PFAS, a group of synthetic chemicals in a wide range of products, is causing a growing concern. Despite their widespread use, some PFAS compounds have been found to degrade into potentially harmful byproducts like PFAS-methyl tetrahydrofuran. What's more alarming is that these chemicals have infiltrated our drinking water sources, even in areas with high water tables. This is why it's crucial to understand effective methods for removing PFAS from water. What should you do if you suspect that there’s a problem with your water? Check the source of the water, test it, and treat it if necessary.
Follow these steps to remove PFAS from drinking water.
Test Your Water
Although knowing how to remove contaminants is essential, it’s even more important to understand how to test your water for contamination. A water test kit can help you determine whether there are contaminants in your water and whether they are at a dangerous level. You can purchase water test kits at grocery stores, hardware stores, and online retailers. Generally, these kits come with the standard set of tests for a home water filtration system, but they also often include tests for specific contaminants. Use these tests to determine whether your water is safe to drink. If your water contains contaminants, remove them from your water source. This can be done by digging a more bottomless well, installing a water filtration system, or getting a water purification system. If your water does not contain contaminants, you don’t need to do anything except continue drinking your water.
Check the Source
Understanding the source of your water is a crucial step in addressing contamination. Whether you have a well or a water treatment system, knowing where your water comes from can provide valuable insights. By tracing the water's journey from the source, you can determine if contamination occurs upstream. This knowledge is essential for well owners, who might overlook the significance of understanding their water source. If you find contamination at the source, you can address it immediately, such as reducing the distance between the source and your dwelling or seeking alternative, uncontaminated sources.
Read More
Topics:
water treatment issues,
water quality,
odor control,
water treatment,
advanced treatment solutions,
Chemical Odor,
Safe drinking water,
RO system,
filters,
Filter Media,
residential well water systems,
DeLoach Industries, Inc.,
backwash,
Carbon Filter,
Micron Filter,
Drinking Water,
Clean Water,
Contaminated Water,
Water Source,
Sediment Filter,
PFA's,
Water Test,
Water Test Kit
Requires an application commonly referred to as either “Degasification” or "Decarbonation" and it requires the use of a piece of water treatment equipment called either a “degasifier” or a “decarbonator”.
Both of these are similar in nature and are designed for Carbon Dioxide (CO2) removal from the incoming water. A properly designed decarbonator can remove 99.99% of the free carbon dioxide gas that is present in the water stream. One of the primary reasons for utilizing a decarbonator or degasifier for the removal of carbon dioxide gas is the raise the pH of the water without the need to add caustic. resulting in high-purity water.
The other reason is the remove the CO2 prior to treating the water with Ion Exchange which utilizes Anion or Cation resins to reduce the regeneration cycles for the resin beds. High concentrations of CO2 consume the ion charge within the resins and require more frequent regeneration cycles. The difference between anion and cation resins is that one is positively charged (anion) and the other is negatively charged (cation), cation resins, attract positive ions with their negative charge.
The term decarbonation describes the process of the removal of suspended gas or the conversion of carbonic acids into free Carbon Dioxide. Carbonic Acid (H2CO3) is stable at normal ambient anhydrous conditions. However, Carbonic Acid decomposes when not stable and in the presence of any water molecules to form carbon dioxide (CO2). The Carbonic acid breaks down when present in water and it is converted to a gas based upon certain conditions. It is common to have CO2 present in water requiring a decarbonation process when utilizing certain types of water filtration such as membrane filtration with reverse osmosis or it can be present when the need to adjust pH is required. When removing (CO2) the process is often referred to as “Decarbonation”, when removing (H2S) Hydrogen Sulfide the process is often referred to as “Degasification”.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier
The Basics of Water Decarbonation
and the removal of carbon dioxide (CO2). The need to remove (CO2) is essential in most Aquaculture, Municipal, Industrial, and Food & Beverage Processes To understand you must familiarize yourself with Henry’s Law.
Henry's Law defines the method and proportional relationship between the amount of a gas in a solution in relation to the gas's partial pressure in the atmosphere. Often you will see and hear various terms like degasification, decarbonation, aeration, and even air stripping when discussing the removal of dissolved gases and other convertible elements from water. Understanding the impacts that Carbon Dioxide (CO2) can have on both equipment and aquatic life provides the basic reasons why the need to decarbonate water, exists. Carbon Dioxide (CO2) can exist naturally in the raw water supply or be the result of ph control and balance. In either case, the process called Decarbonation or Degasification provides the most cost-effective and efficient manner to reduce or tally remove (CO2) from the water. In addition to Carbon Dioxide (CO2), water can contain a variety of other contaminants that may impact the removal efficiency of the Carbon Dioxide. A variety of elements as well as dissolved gases such as oxygen, nitrogen, and carbon dioxide (CO2). A full analytical review of the water chemistry is required to properly design and size the “Water Treatment” process.
Breaking the bonds in water releases a dissolved gas
such as carbon dioxide (CO2) you must change the conditions of the vapor pressure surrounding the gas and allow the gas to be removed. There are many variables to consider when designing or calculating the “means and methods” of the removal of carbon dioxide (CO2). When I refer to the means and methods. I am referring to the design of a decarbonator and its components. The means equals the size and type (Hydraulic load) of the decarbonator and the “method” equals the additional variables such as the cubic foot of airflow (CFM) and “Ratio” of the air to water to accomplish the proportional condition needed to remove the carbon dioxide (CO2).
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
removal of CO2 from water
One of the most pressing issues that we face today is the phenomenon of red tide.
Red tide is algae bloom that can cause serious harm to marine life and humans.
In this article, I will explore what red tide is, what causes it, and its impact on Florida. Most importantly, the role of wastewater treatment in helping to prevent it.
What is Red Tide?
Red tide is a natural phenomenon that occurs when certain species of algae grow out of control. These algae produce toxins that harm marine life and humans. The term "red tide" comes from the reddish-brown color that the water takes on when the algae bloom. The bloom can happen in any part of the world in warm, coastal waters.
What Causes Red Tide?
Various factors cause these harmful algae to bloom.
- Changes in water temperature
- Nutrient pollution
- Ocean currents
The most common cause is nutrient pollution. Nutrient pollution occurs when excess nutrients like nitrogen and phosphorus enter the water. These nutrients can come from agricultural runoff, sewage from residential drain fields, inefficient wastewater treatment plants, septic tanks, and fertilizer.
Red Tide Blooms in Florida - History and Impact.
Florida has a long history of deadly algae blooms. The state experiences red tide almost every year, lasting for months.
It devastates the state's marine life, including fish, sea turtles, and dolphins. The algae produce toxins that can kill these animals. The dead fish can wash up on shore, causing beachgoers an unpleasant odor and an eyesore.
How Do These Microscopic Algae Affect Marine Life?
It affects marine life in a variety of ways. The algae's toxins can cause respiratory problems and neurological issues.
With most coastal outbreaks, the loss of marine life is significant. It produces toxins and decreases oxygen levels, another contributing factor to marine animal fatalities. Red tide disrupts the entire ecosystem and has been increasing in both frequencies of outbreaks as well as areas impacted.
How Does Red Tide Impact Humans?
Read More
Topics:
water treatment issues,
water treatment,
wastewater,
DeLoach Industries,
water process system,
red tide,
wastewater treatment plants,
red tide in florida,
wastewater treatment system,
cause of red tide,
water temperature,
marine life,
wastewater treatment infrastructure,
benefits of wastewater treatment,
water treatment standards
If you’ve been reading the news lately, you know nanoparticles are not so great. In everything from cosmetics to water filters, nanoparticles have been shown to cause various health problems. But what exactly are nanoparticles, and how can you protect yourself from their harmful effects? Let’s answer these questions and more with this quick guide on removing nanoparticles from your drinking water.
Read More
Topics:
water treatment issues,
water quality,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
safety,
Safe drinking water,
Global,
distillation,
RO membrane,
RO system,
particulate matter,
filters,
municipal water systems,
residential well water systems,
DeLoach Industries, Inc.,
Drinking Water,
Clean Water,
Water Test,
Water Test Kit,
DeLoach Industries,
technology,
minerals,
temperature,
nanoparticles,
Cosmetics,
Nano,
make-up,
organ function,
contaminants,
pressure filters,
reverse osmosis,
carbon filters,
UV filters,
activated carbon
Odor control in a manufacturing facility is essential.
It prevents potential health risks and discomfort caused by the spread of chemicals, vapors, and fumes. Additionally, excessive vapors can hinder the efficiency of exhaust and natural ventilation systems.
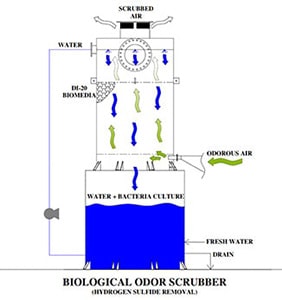
One effective solution for addressing odor issues is the installation of an Odor Control Scrubber Tower. These towers are part of the ventilation system in manufacturing plants and chemical processing facilities.
Odor control scrubbers help to remove noxious fumes and odors from exhaust and air streams. This is an effective way to improve air quality. This process involves utilizing an activated carbon filter and an ionic air filter
Key Considerations for Installing an Odor Control Scrubber Tower:
Health and Safety of Workers:
Industrial environments pose risks of exposure to hazardous fumes and gases for workers. Unhealthy odors emitted in high concentrations can jeopardize their well-being and safety. In some cases, these gases may even be combustible, adding an extra level of danger.
Odor control scrubber towers remove gases from the contaminated air, ensuring a safe working environment. These towers reduce the risk of health issues such as nausea, headaches, allergy symptoms, eye irritation, and loss of consciousness. This helps maintain worker productivity and prevents sickness caused by toxic fumes and gases.
Read More
Topics:
water treatment issues,
water quality,
odor control,
water treatment,
water distribution system,
advanced treatment solutions,
biological scrubber,
water plant,
safety,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
caustic,
Safe drinking water,
wastewater,
gases,
Biological Odor Control Scrubber,
Biological odor control,
what is a scrubber,
municipal water systems,
DeLoach Industries, Inc.,
Clean Water,
Industrial Odor Control