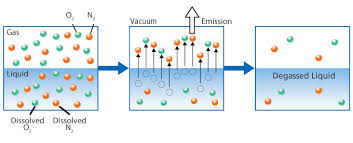
Degasification and decarbonation are essential processes in water treatment that play a crucial role in improving water quality.
Read More
Topics:
degasification,
hydrogen sulfide (H2S),
Decarbonation,
dissolved gases,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
co2 dissolved in water,
degassed water,
decarbonation of water,
DeLoach Industries, Inc.,
hydrogen sulfide molar mass,
DeLoach Industries,
carbon filters,
removing hydrogen sulfide in water,
hydrogen sulfide gas,
dissolved oxygen
Requires an application commonly referred to as either “Degasification” or "Decarbonation" and it requires the use of a piece of water treatment equipment called either a “degasifier” or a “decarbonator”.
Both of these are similar in nature and are designed for Carbon Dioxide (CO2) removal from the incoming water. A properly designed decarbonator can remove 99.99% of the free carbon dioxide gas that is present in the water stream. One of the primary reasons for utilizing a decarbonator or degasifier for the removal of carbon dioxide gas is the raise the pH of the water without the need to add caustic. resulting in high-purity water.
The other reason is the remove the CO2 prior to treating the water with Ion Exchange which utilizes Anion or Cation resins to reduce the regeneration cycles for the resin beds. High concentrations of CO2 consume the ion charge within the resins and require more frequent regeneration cycles. The difference between anion and cation resins is that one is positively charged (anion) and the other is negatively charged (cation), cation resins, attract positive ions with their negative charge.
The term decarbonation describes the process of the removal of suspended gas or the conversion of carbonic acids into free Carbon Dioxide. Carbonic Acid (H2CO3) is stable at normal ambient anhydrous conditions. However, Carbonic Acid decomposes when not stable and in the presence of any water molecules to form carbon dioxide (CO2). The Carbonic acid breaks down when present in water and it is converted to a gas based upon certain conditions. It is common to have CO2 present in water requiring a decarbonation process when utilizing certain types of water filtration such as membrane filtration with reverse osmosis or it can be present when the need to adjust pH is required. When removing (CO2) the process is often referred to as “Decarbonation”, when removing (H2S) Hydrogen Sulfide the process is often referred to as “Degasification”.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier
The Basics of Water Decarbonation
and the removal of carbon dioxide (CO2). The need to remove (CO2) is essential in most Aquaculture, Municipal, Industrial, and Food & Beverage Processes To understand you must familiarize yourself with Henry’s Law.
Henry's Law defines the method and proportional relationship between the amount of a gas in a solution in relation to the gas's partial pressure in the atmosphere. Often you will see and hear various terms like degasification, decarbonation, aeration, and even air stripping when discussing the removal of dissolved gases and other convertible elements from water. Understanding the impacts that Carbon Dioxide (CO2) can have on both equipment and aquatic life provides the basic reasons why the need to decarbonate water, exists. Carbon Dioxide (CO2) can exist naturally in the raw water supply or be the result of ph control and balance. In either case, the process called Decarbonation or Degasification provides the most cost-effective and efficient manner to reduce or tally remove (CO2) from the water. In addition to Carbon Dioxide (CO2), water can contain a variety of other contaminants that may impact the removal efficiency of the Carbon Dioxide. A variety of elements as well as dissolved gases such as oxygen, nitrogen, and carbon dioxide (CO2). A full analytical review of the water chemistry is required to properly design and size the “Water Treatment” process.
Breaking the bonds in water releases a dissolved gas
such as carbon dioxide (CO2) you must change the conditions of the vapor pressure surrounding the gas and allow the gas to be removed. There are many variables to consider when designing or calculating the “means and methods” of the removal of carbon dioxide (CO2). When I refer to the means and methods. I am referring to the design of a decarbonator and its components. The means equals the size and type (Hydraulic load) of the decarbonator and the “method” equals the additional variables such as the cubic foot of airflow (CFM) and “Ratio” of the air to water to accomplish the proportional condition needed to remove the carbon dioxide (CO2).
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
removal of CO2 from water
Basics of water decarbonation for dissolved organic carbon.
The water treatment industry continues to develop and evolve. Over the past two decades, there have been many new developments in technology and even more refinement in existing technologies such as "Degasification". The evolution and advancement of water treatment have been driven by the constantly increasing demand from an increase in population that demand cost-effective solutions and recognition to improve safety with the implementation of NSF 61 standards.
All human cultures on our planet share a single commonality: the dependency on water to survive.
Many existing technologies, such as "Degasification," have evolved with higher efficiency to meet the demand changes and provide safety to consumers and the systems. Degasification refers to the removal of dissolved gases from liquids, and the science to degasify water is based upon a chemistry equation known as "Henry's Law". The "proportionality factor" is called Henry's law constant" and was developed by William Henry in the early 19th century. Henry's Law states that "the amount of dissolved gas is proportional to its partial pressure in the gas." The most "cost" effective method to perform degasification is with the packed vertical tower called a "Degasifier” or “Decarbonator.”
The key words in this previous sentence for owners, operators, and engineers to focus on is "the most cost-effective" as there is no other process more cost-effective at removing dissolved gases at the lowest cost than using a Degasifier or decarbonator. The process of degasification is simple enough to understand. Water is pumped to the top of a vertically constructed tower, where it first enters the tower through some type of distribution system at the same time, there is a cross-current air flowing up from the bottom by a blower located at the bottom of the tower, and the air encounters the water and is exhausted at the top of the tower through an exhaust port. There are various types of distribution systems, and we will explore these in later discussions. Once the water enters the top of the tower and passes through the distribution system, it then travels by gravity downward. The next thing the water encounters is some type of media packing. There are various forms of media packing offered in the degasification industry, and each type can offer higher performance or have the ability to deter fouling. The selection of the type, size, and volume is where the “experience, engineering, and understanding of each application” comes into play.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
NSF/ANSI 61,
hydrogen sulfide (H2S),
media packing,
pH levels,
scaling,
caustic,
Decarbonation,
Safe drinking water,
dissolved gases,
carbon dioxide,
decarbonator,
boiler system,
degasifier,
carbonic acid,
H2S Degasifier,
Dissolved organic Carbon,
co2 dissolved in water
In the production and purification of water for industry
there are many types of different processes available to remove harmful minerals and gases from the water stream but the most effective process and most cost-effective from both a capital investment and operational cost is a “Forced Draft Degasification System” (Degasifier).
Degasification is used in a wide range of water processes for industrial and municipal applications which extend from the production of chemicals to the production of semiconductors and in all applications the need to remove contaminants from the water and dissolved gases is key to achieving the end results needed in the industrial water process. Water from the ground often contains elements such as calcium carbonate, manganese, iron, salts, hydrogen sulfide, and sulfur just to name a few of the basic contaminants and these naturally occurring elements can cause serious damage and consequences to process equipment such as boiler systems, piping, membranes, and cation and anion exchange resins used in the demineralization process.
Calcium carbonate can dissolve in water under certain pH ranges forming carbonic acid and releasing carbon dioxide (CO2) gases. These gases are not only very corrosive to equipment like boiler feed systems and boiler tubes but also attack the actual resin beds found in cation and anion softening and demineralization system causing an increase in regeneration and chemical consumption and resin bed replacement.
By incorporating a Force Draft Degasification system you can remove dissolved gasses
like CO2 and hydrogen sulfide (H2S) to as low as 99.999% and improve the cation and anion system performance, extend the resin bed life, and lower the operating cost of the water treatment process.
Quite often Forced Draft Degasification is utilized “post” treatment to also remove newly formed dissolved gases prior to entering the boiler feed system to prevent corrosion damage within the tubes and feed system and pumps. These gases are easily removed with the forced draft degasifier at a much lower cost than chemical additives or liquid cell degasification that requires higher capital cost and much higher operating cost.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
iron oxidation,
water treatment,
water distribution system,
aluminum,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
calcium carbonate,
media packing,
pH levels,
Langilier index (LSI),
Decarbonation,
ION Exchange Resin,
dissolved gases,
feed water,
De-Aeration,
wastewater,
carbon dioxide,
decarbonator,
degasifier,
carbonic acid,
H2S Degasifier
To enhance and control the production and quality of seafood grown and harvested.
The industry increasingly focuses on constructing in-house aquaculture fish farms, commonly called aqua farming. The most popular species of aqua farming continue to be salmon, tilapia, catfish, and carp. Increased interest in the United States has developed aqua farming facilities in southern Florida with favorable climate and water conditions.
When considering several types of fish species to grow for harvest, it is important to remember the need to control the water quality. If the aqua farm is intended to utilize man-made tanks, they will depend upon a constant flow of incoming water. If the aqua farm focuses on salmon, the water quality and temperature play a major role in the operation's mortality rates and production yields.
Having water with too high of hydrogen sulfide, carbon dioxide, total Organic carbons, and even turbidity can increase mortality rates among the younger fish species and is especially critical to salmon.
Having high levels of metals
Such as Iron that is identified as either “ferric” (Fe-) or “ferrous” (FE+2) and is naturally occurring within the Florida waters and other parts of the US will cause significant damage to young salmon species because the metal accumulates within the gills of the fish causing suffocation. Other metals are also detrimental to fish, including copper, aluminum, arsenic, cadmium, chromium, Lead, manganese, and mercury, to name a few, and the water quality must be evaluated and tested in the early stages of design to anticipate the required types of process systems needed.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
Decarbonation,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
carbonic acid,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
Water Treatment
When planning and designing a man made on land aquaculture or pisciculture facility.
The most important key element is the quality of the water. For operations developing in Florida or the Caribbean it is important to remember that water quality varies in Florida and other states in the US and typically requires some type of water treatment. For fresh and salt water land based farms that utilize tanks located inside of a building the water needs to be treated and pure from any naturally occurring contaminants such as hydrogen sulfide (H2S), iron (Fe+), and even carbon dioxide (CO2).
The most cost effective way to treat incoming water for aquaculture farming and remove hydrogen sulfide, iron, and lower carbon dioxide is the use of a “degasification” tower. A degasification tower or degasifier is a piece of process equipment. Degasifiers can also be referred to as a “decarbonator” or “air stripper” or even “aeration tower”. The degasification tower is a vertical column designed to remove certain types of contaminants by “stripping” the molecules of converted gases and expelling them from the water as a gas. The science is based upon “Henry’s Law” and it relies upon the disproportionate varying vapor pressures of gases.
If the incoming raw water contains levels of sulfides or hydrogen sulfide gases it is recommended to remove the hydrogen sulfide to improve the water quality and reduce the risk of the development and formation of bacteria that can thrive on the Sulfur. In addition hydrogen sulfide is corrosive and will cause harm to other components within the process if left untreated. It is important to adjust the pH of the raw feed water prior to degasification to ensure full conversion of the sulfides into hydrogen sulfide gas (H2S) to enable the degasification process to perform and remove up to 99.99% of the harmful contaminants without adding additional chemicals. This saves money and improves quality of the product!
Read More
Topics:
water quality,
degasification,
pH levels of water,
water treatment,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
Decarbonation,
Caribbean,
carbon dioxide,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
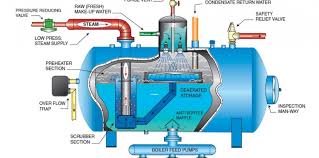
In the United States manufacturing industry, an astonishing 400 million gallons of water per day (MGD) is consumed to generate steam.
Out of this amount, approximately 60 MGD is sent to blow-down drains, while another 300 MGD is used for direct injection of steam. The common denominator in all of these processes is the need for purified and treated water. Without proper treatment, manufacturers would face frequent shutdowns and increased capital expenditure, significantly impacting their cost of goods. One effective method of water treatment to protect boilers is through degasification and deaeration.
Degasification towers play a crucial role in removing harmful gases such as hydrogen sulfide (H2S), carbon dioxide (CO2), and often dissolved oxygen (DO). The elimination of these corrosive gases is vital for enhancing the lifespan and efficiency of boiler systems. If these gases are allowed to remain in the boiler feed water, particularly carbon dioxide (CO2), it can lead to disastrous consequences, including higher operating costs and reduced system longevity. Carbon dioxide (CO2) can convert into carbonic acid, creating a corrosive environment for the boiler and other critical components. In cases where an ion exchange process is implemented prior to the boiler, the presence of carbon dioxide (CO2) can drastically increase regeneration costs as the resins are consumed. By removing carbon dioxide (CO2), the life of the resin is extended, and the pH of the water is elevated, reducing the need for additional chemicals and further lowering operating costs.
Read More
Topics:
water treatment issues,
degasification,
iron oxidation,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
Decarbonation,
ION Exchange Resin,
feed water,
De-Aeration,
steam generation,
steam generating boilers,
carbon dioxide,
steam,
decarbonator,
boiler system,
degasifier,
gases,
RO membrane,
carbonic acid,
RO system,
H2S Degasifier,
Boiler feed water
Optimizing Steam Process Water Systems with Degasification Towers
Steam process water systems are integral to various industrial operations, where water is heated and converted into steam. However, ensuring the efficiency and longevity of these systems requires a comprehensive understanding of water chemistry and the implementation of proper treatment methods. In particular, the removal of dissolved gases, such as hydrogen sulfide (H2S), carbon dioxide (CO2), and dissolved oxygen (O2), is crucial. This blog post will delve into the significance of degasification towers in steam process water systems, emphasizing their role in preventing corrosion, enhancing equipment performance, and maintaining water quality in your water and wastewater systems.
The Importance of Removing Dissolved Gases
Dissolved gases in steam process water systems can have detrimental effects on boilers and other critical components. Allowing gases like carbon dioxide (CO2) to remain in the water leads to the formation of carbonic acid, creating a corrosive environment. This corrosion can damage the boiler and reduce its lifespan. Additionally, dissolved gases can impair the efficiency of the system, affecting heat transfer and leading to reduced performance.
Read More
Topics:
De-Aeration,
carbon dioxide,
oxygen,
steam,
decarbonator,
degasifier,
carbonic acid
Protecting Your Pharmaceutical Water: Ensuring Quality and Efficiency in Water Treatment
In the pharmaceutical industry, the removal of dissolved gases from water is a critical step in the water treatment process. However, it is essential to select the appropriate method of removing these gases, as the wrong choice can have detrimental effects on vital process water equipment such as steam boilers and distillation columns. Failure to address high levels of carbon dioxide (CO2) in the water can lead to the formation of carbonic acid, which corrodes and damages both the steam boiler tubes and distillation columns. To mitigate these risks, the implementation of a degasification tower or "Degasifier" is crucial, as it effectively removes dissolved gases like hydrogen sulfide (H2S) and carbon dioxide (CO2) to acceptable levels below 7 parts per billion (ppb).
Utilizing a degasification tower offers a cost-effective solution to reduce and eliminate gases in the water stream. In comparison, alternative methods such as reverse osmosis (RO) membranes require additional steps, including pH adjustment, to achieve similar results. The conversion of carbon dioxide (CO2) into carbonates can result in increased membrane fouling and elevated capital costs for the RO system. By implementing a degasification system, businesses can achieve optimal performance, minimize membrane fouling, and benefit from cost savings in both capital and operational expenses.
Read More
Topics:
degasification,
water treatment,
hydrogen sulfide (H2S),
dissolved gases,
pharmaceutical water,
carbon dioxide,
degasifier,
gases,
RO membrane,
carbonic acid,
RO system