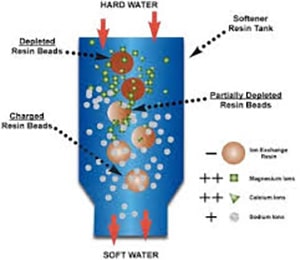
Basics of water decarbonation for dissolved organic carbon.
The water treatment industry continues to develop and evolve. Over the past two decades, there have been many new developments in technology and even more refinement in existing technologies such as "Degasification". The evolution and advancement of water treatment have been driven by the constantly increasing demand from an increase in population that demand cost-effective solutions and recognition to improve safety with the implementation of NSF 61 standards.
All human cultures on our planet share a single commonality: the dependency on water to survive.
Many existing technologies, such as "Degasification," have evolved with higher efficiency to meet the demand changes and provide safety to consumers and the systems. Degasification refers to the removal of dissolved gases from liquids, and the science to degasify water is based upon a chemistry equation known as "Henry's Law". The "proportionality factor" is called Henry's law constant" and was developed by William Henry in the early 19th century. Henry's Law states that "the amount of dissolved gas is proportional to its partial pressure in the gas." The most "cost" effective method to perform degasification is with the packed vertical tower called a "Degasifier” or “Decarbonator.”
The key words in this previous sentence for owners, operators, and engineers to focus on is "the most cost-effective" as there is no other process more cost-effective at removing dissolved gases at the lowest cost than using a Degasifier or decarbonator. The process of degasification is simple enough to understand. Water is pumped to the top of a vertically constructed tower, where it first enters the tower through some type of distribution system at the same time, there is a cross-current air flowing up from the bottom by a blower located at the bottom of the tower, and the air encounters the water and is exhausted at the top of the tower through an exhaust port. There are various types of distribution systems, and we will explore these in later discussions. Once the water enters the top of the tower and passes through the distribution system, it then travels by gravity downward. The next thing the water encounters is some type of media packing. There are various forms of media packing offered in the degasification industry, and each type can offer higher performance or have the ability to deter fouling. The selection of the type, size, and volume is where the “experience, engineering, and understanding of each application” comes into play.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
NSF/ANSI 61,
hydrogen sulfide (H2S),
media packing,
pH levels,
scaling,
caustic,
Decarbonation,
Safe drinking water,
dissolved gases,
carbon dioxide,
decarbonator,
boiler system,
degasifier,
carbonic acid,
H2S Degasifier,
Dissolved organic Carbon,
co2 dissolved in water
DeLoach Industries made history in 1977 at the City of Cape Coral Florida water treatment plant with its large scale “degasification towers” connected to what was to become the first municipal water treatment facility in the United States to deploy the use of reverse osmosis on a large-scale production municipal treatment plant.
The Cape Coral water treatment plant for came online in 1977 and produced 3 million gallons of water per day (GPD) or 11.35 liters of purified and treated water utilizing the “reverse osmosis” process. By 1985 the plant had expanded as it kept up with growth to produce 15 million gallons per day making it at the time the world’s largest “reverse osmosis” water treatment plant facility.
Read More
Topics:
water quality,
pH levels of water,
water treatment,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
scaling,
chlorine,
caustic,
Decarbonation,
wastewater,
carbon dioxide,
degasifier,
RO membrane,
RO system,
H2S Degasifier
Avoid problems with calcium chlorite and corrosive gasses with your odor control scrubber.
When planning or designing an odor control system, one should pay close attention to several key variables that can cause havoc on a chemical odor control scrubber when trying to treat hydrogen sulfide or ammonia gases. The need for odor control occurs in many different forms. It is essential to understand the process that is creating the odorous or corrosive gas and the need for odor control & air emissions treatment.
First, begin to identify
all the potential obstacles that may creep up later after the chemical odor or corrosive gas control system goes online, like acid or caustic consumption. For example, chemical odor control systems designed for water treatment for the municipal industry are typically needed and attached to a degasification or decarbonation process, often needed to treat hydrogen sulfide (H2S). However, designers often may not pay close enough attention to the type of water process available for “make-up” water for the chemical scrubber. The addition of caustic can create scaling or fouling. This unknown variable of the makeup water quality can lead to a complete tower shutdown if the chemical scrubber distribution and media bed scales or fouls. The most commonly used chemicals for a hydrogen sulfide (H2S) scrubber are either chlorine in the form of sodium hypochlorite or caustic in the form of caustic soda. Both of these chemicals are common to a water treatment facility and are already in place to adjust and control pH.
The makeup water plays a significant role in the operation of a chemical scrubber.
When water containing high hardness levels is used as the source for the makeup water, your chemical scrubber can become fouled, and scaling can occur in a matter of hours, depending on the alkalinity and salts within the water. Solidification can occur from the scaling when combining sodium hypochlorite and raw feed water at specific pH ranges and these ranges are usually the range needed to achieve peak performance. Calcium chloride will form, and your chemical odor control scrubber will become a solid chunk of calcium chlorite making, making the ability for water or air to pass freely through the media packing next to impossible. No matter what type of media packing is utilized in the odor control or gas scrubber, it can foul and scale if the water chemistry is incorrect. Trust me when I say “been there and done that”! I have seen operators who have allowed a chemical scrubber to become out of balance with pH control and completely solidify the tower column to the degree that neither air nor water passage is possible. The problem can still occur with ammonia scrubbers but are different with different sets of parameters.
Read More
Topics:
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
calcium carbonate,
media packing,
pH levels,
Alkalinity,
Langilier index (LSI),
scaling,
chlorine,
caustic,
ION Exchange Resin,
Safe drinking water,
dissolved gases,
De-Aeration,
carbon dioxide,
oxygen,
degasifier,
gases,
H2S Degasifier,
calcium chlorite
Extending the life of your ion exchange resin in boiler feed water applications
Did you know that when producing steam, it is most effectively accomplished by utilizing a decarbonation process? Decarbonation and Degasification is the most economical way to process fluent water pre-Ion exchange treatment through a vertically packed tower called a decarbonator, often called a degasifier. This is the most economical method to remove carbon dioxide (CO2) and Hydrogen Sulfide (H2S) to prevent the formation of carbonic acid. Other corrosive conditions are Degasification and Decarbonation, which will extend the life of the ion exchange resin. If CO2 levels remain high in the inlet feed water to the ion exchange system, the resin beds, whether cation or anion, will require more frequent regeneration, and your chemical usage and cost will rise.
In industrial applications, it’s easy to overlook.
Often, there is insufficient focus on selecting the right decarbonation or degasification system to ensure that the process water treatment system performs at the highest optimal level. For water filtration, when the primary process is membrane filtration, often referred to as “reverse osmosis, " too little attention is given to properly removing CO2 from the process to lower the pH and adjust the alkalinity.
Read More
Topics:
water treatment issues,
scaling,
Decarbonation,
ION Exchange Resin
Maintaining Water Quality: Key to Effective Chemical Odor Control Treatment Systems
Read More
Topics:
odor control,
calcium carbonate,
Chemical Odor,
media packing,
Langilier index (LSI),
scaling,
chlorine,
caustic
Water treatment towers and storage tanks are high places that require special precautions when entering. While the majority of people who enter these locations for work can be trusted, there are some hazards that make it more important than usual to follow safety procedures.
These locations can get very hot and humid, and can also be filled with harmful chemicals and microorganisms that can cause serious health issues if inhaled or absorbed through the skin. Therefore, the general standard for workplace safety is much higher when entering locations like these.
Make sure you have read and understood the following information about safety when entering a water treatment plant. It will help you understand how to stay safe and protect yourself from harm when entering a water treatment plant. normal installation, maintenance, or even emergency repairs, it is often required to enter into a water treatment tower (degasifier, air stripper, decarbonator, or clear well/ storage tank). When this occurs, full safety protocols should be followed at all times, in accordance with OSHA regulations. A tower or tank B classification is a "Confined Space" location. For more information visit the OSHA confined space regulations page.
In addition, there are other safety risks that an operator or technician can be exposed to while inside these types of closed locations. The risk can come from fumes of hydrogen sulfide (H2S), chlorine from an injection line, or a lack of oxygen O2. A proper confined space permit should be prepared and only technicians with proper training and certifications should enter into these types of confined spaces.
Read More
Topics:
water treatment issues,
water quality,
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
safety,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
media packing,
scaling,
caustic,
Safe drinking water,
dissolved gases,
wastewater,
carbon dioxide,
degasifier,
gases,
Ammonia,
what is a scrubber,
Hydrogen Sulfide formula,
Deagasification,
Filter Media,
DeLoach Industries, Inc.,
Drinking Water,
Clean Water,
Contaminated Water,
OSHA