Requires an application commonly referred to as either “Degasification” or "Decarbonation" and it requires the use of a piece of water treatment equipment called either a “degasifier” or a “decarbonator”.
Both of these are similar in nature and are designed for Carbon Dioxide (CO2) removal from the incoming water. A properly designed decarbonator can remove 99.99% of the free carbon dioxide gas that is present in the water stream. One of the primary reasons for utilizing a decarbonator or degasifier for the removal of carbon dioxide gas is the raise the pH of the water without the need to add caustic. resulting in high-purity water.
The other reason is the remove the CO2 prior to treating the water with Ion Exchange which utilizes Anion or Cation resins to reduce the regeneration cycles for the resin beds. High concentrations of CO2 consume the ion charge within the resins and require more frequent regeneration cycles. The difference between anion and cation resins is that one is positively charged (anion) and the other is negatively charged (cation), cation resins, attract positive ions with their negative charge.
The term decarbonation describes the process of the removal of suspended gas or the conversion of carbonic acids into free Carbon Dioxide. Carbonic Acid (H2CO3) is stable at normal ambient anhydrous conditions. However, Carbonic Acid decomposes when not stable and in the presence of any water molecules to form carbon dioxide (CO2). The Carbonic acid breaks down when present in water and it is converted to a gas based upon certain conditions. It is common to have CO2 present in water requiring a decarbonation process when utilizing certain types of water filtration such as membrane filtration with reverse osmosis or it can be present when the need to adjust pH is required. When removing (CO2) the process is often referred to as “Decarbonation”, when removing (H2S) Hydrogen Sulfide the process is often referred to as “Degasification”.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier
The Basics of Water Decarbonation
and the removal of carbon dioxide (CO2). The need to remove (CO2) is essential in most Aquaculture, Municipal, Industrial, and Food & Beverage Processes To understand you must familiarize yourself with Henry’s Law.
Henry's Law defines the method and proportional relationship between the amount of a gas in a solution in relation to the gas's partial pressure in the atmosphere. Often you will see and hear various terms like degasification, decarbonation, aeration, and even air stripping when discussing the removal of dissolved gases and other convertible elements from water. Understanding the impacts that Carbon Dioxide (CO2) can have on both equipment and aquatic life provides the basic reasons why the need to decarbonate water, exists. Carbon Dioxide (CO2) can exist naturally in the raw water supply or be the result of ph control and balance. In either case, the process called Decarbonation or Degasification provides the most cost-effective and efficient manner to reduce or tally remove (CO2) from the water. In addition to Carbon Dioxide (CO2), water can contain a variety of other contaminants that may impact the removal efficiency of the Carbon Dioxide. A variety of elements as well as dissolved gases such as oxygen, nitrogen, and carbon dioxide (CO2). A full analytical review of the water chemistry is required to properly design and size the “Water Treatment” process.
Breaking the bonds in water releases a dissolved gas
such as carbon dioxide (CO2) you must change the conditions of the vapor pressure surrounding the gas and allow the gas to be removed. There are many variables to consider when designing or calculating the “means and methods” of the removal of carbon dioxide (CO2). When I refer to the means and methods. I am referring to the design of a decarbonator and its components. The means equals the size and type (Hydraulic load) of the decarbonator and the “method” equals the additional variables such as the cubic foot of airflow (CFM) and “Ratio” of the air to water to accomplish the proportional condition needed to remove the carbon dioxide (CO2).
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
removal of CO2 from water
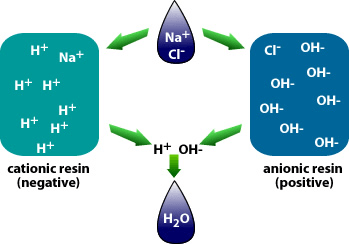
Water demineralization is also called deionization and is a process known as “Ion Exchange.”
In simple terms, water demineralization is “Water Purification.” The process involves removing dissolved ionic mineral solids from a feed-water process, typically for “Industrial” water applications. Still, it can also be utilized to remove dissolved solids from a water process for “Aquaculture,” “Food and Beverage,” and the “Municipal” markets.
Why is demineralization utilized? It can remove dissolved solids to near distilled water quality at a much lower capital and operational cost than other treatment processes such as membrane softening (Reverse Osmosis). Demineralization applies the science known as “Ion Exchange,” which attracts negative and positive charged ions and allows either to attach themselves to a negative ion depending on their respective current negative or positive charge during what is known as a resin cycle. In other technical articles, we will explore and go into more specific details on the science of the ion exchange process. Water that has dissolved salts and minerals has ions, either negatively charged ions known as “Anions” or positively charged ions known as “Cations.” To treat the water and remove these contaminants, the ions in the water are attracted to counter-ions, which have a negative charge. In a demineralization treatment process, there are pressure vessels that hold resin beads which are typically made of plastic. The beads are made from a plastic material with an ionic functional group that allows them to hold and maintain an electrostatic electrical charge. Some of these resin groups are negatively charged, referred to as “Anion” resins, while others hold a positive charge and are called “Cations” resins.
There are different applications to apply Ion exchange technologies, which is why you will often hear different terminology interchanged like deionization and demineralization. The raw water quality and the specific application will dictate the type of ion exchange process needed. For example, if the water contains a high level of hardness, the water will most likely contain Ca2+ or Mg2+ dissolved solids possessing a positive charge. To replace these hard ions, it is typical to utilize a resin bed with a salt ion like Na+. As the water passes over the resin bead material within the pressure vessel. The hard ions are replaced with the salt ion; therefore, all the hardness within the water is removed. However, the water will now contain a higher concentration of sodium ions, and this must be considered during the evaluation and selection process of the type of resin material to utilize for the specific application. If the water application requires high purity and the removal of as many solids as possible, then the term or process selected is referred to as demineralization.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
media packing,
Decarbonation,
ION Exchange Resin,
decarbonator,
degasifier,
RO system,
H2S Degasifier,
Aquaculture,
degassed water,
Co2 ph,
removal of CO2 from water,
Deagasification,
decarbonation of water,
hydrogen ion,
particulate matter,
municipal water systems,
industrial facilities,
automated control systems,
Ion exchange,
cations,
anions
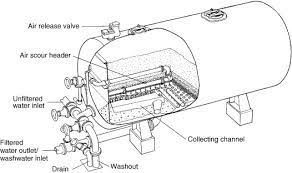
Industrial water systems use water filters to reduce the level of solids in water from:
- Industrial
- Semiconductor
- Manufacturing
- Refining
- Oil and Natural Gas Production Processes
The wastewater may contain harmful chemicals to humans, plants, or animals. Three types of filters are commonly used in industrial settings:
Gravity filters, pressure filters, and constructed wetlands. Pressure filters have two variations: multimedia and higher-pressure micron or cartridge filters. Constructed wetlands or natural filters are not often utilized in industrial processes. Based on the requirements to obtain environmental permits and safeguard the ecosystem.
There are many benefits to pressure filtering systems in industrial wastewater. Pressure filters can remove particles down to 0.3 microns in size. They don't clog up as quickly as other filter types, and it's much faster than other types of filtration methods.
Pressure filtering is also very cost-effective because it uses less energy than other methods. Look no further if you're looking for a high-quality industrial water filter that cuts down on operating costs!
Pressure Filters (Multimedia type) are often used in industrial settings to filter particulates down to 15 microns in size.
They're also very cost-effective due to their energy; pressure filters utilize much less energy than other filtration methods. Pressure filters can include multimedia, a mixture of gravel and sand, multimedia, gravel, sand, and anthracite, or multimedia, which combines gravel, sand, greensand, and anthracite filter media. The variations are dependent on the applications and the need.
Read More
Topics:
water quality,
water treatment,
water plant,
media packing,
ION Exchange Resin,
RO system,
Pressure filter,
Sand filters,
Filter Media,
industrial facilities,
green sand,
Gravity Filters,
Constructed Wetlands
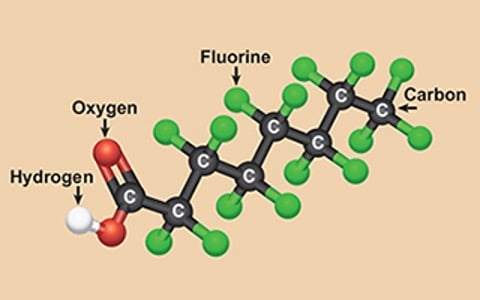
Per- and polyfluorinated substances (PFAS), known as "forever chemicals," have long been utilized in various consumer products due to their exceptional properties.
However, the challenge lies in effectively treating or eliminating PFAS once they enter the environment or water supply. This blog will focus on the technological advancements in removing PFAS and perfluorooctanoic acids (PFOAs) from water sources. By exploring different treatment methods, such as activated carbon absorption, ion exchange resins, and reverse osmosis, and simply avoiding PFOA and PFOS, we can better understand the available options for mitigating these persistent chemicals in water.
Activated Carbon Absorption
One of the earliest technologies employed for PFAS removal is activated carbon absorption. This method involves the use of specially treated carbon materials that effectively adsorb PFAS compounds from water sources. The activated carbon's large surface area and porous structure allow it to trap and retain PFAS molecules. This technology has proven effective in removing PFAS, including PFOAs, from drinking water and environmental sources. However, periodic treatment and regeneration of the activated carbon are necessary to maintain its efficacy.
Read More
Topics:
degasification,
iron oxidation,
water treatment,
advanced treatment solutions,
water plant,
ION Exchange Resin,
Safe drinking water,
wastewater,
degasifier,
RO system,
Deagasification,
PFA's,
technology,
contaminants,
reverse osmosis,
carbon filters,
activated carbon,
removing PFAS & PFOS,
pfas exposure,
health effects of pfas,
nonstick cookware,
wastewater treatment systems,
PFOS,
pfoa regulations,
drinking water standards,
water resistant clothing,
environmental safety
In many water treatment and chemical processes,
it is a requirement to keep track of the pH of the water or product stream. In DeLoach Industries equipment such as degasification systems, or odor control scrubbers, pH measurement is critical to control the chemical reactions happening within the treatment system. PH is an indication of the acidic vs alkaline nature of the fluid. An acidic fluid will have a greater concentration of H+ hydrogen ions, while an alkaline fluid will have a greater concentration of OH- hydroxide ions. This electrochemical nature is used in the construction, reading, and maintenance of electronic pH probes.
PH probes are generally glass and will contain a reference element, and a sensing element. When the pH probe is immersed in the fluid to be measured, the electrical potential difference between the sensing element and the reference element is amplified by electronics and the resulting voltage is used in a calculation to determine pH from differential electron potential. As a pH probe remains in service, ion exchange will slowly change the electrical potential of the sensing element, the reference element, or both. This happens because the hydrogen ions are small enough to travel through the glass sensor body and cause reference potential shifts over time. This is normal behavior for all pH probes and is the reason why pH probes must be periodically calibrated.
Calibration is a process where a pH probe is immersed in a series of standardized stable pH solutions called “buffers”. The standard set of buffers includes a pH 4.0 acidic buffer, a pH 7.0 neutral buffer, and a pH 10.0 alkaline buffer. These buffer solutions are chemically designed to hold a stable pH and are used as a reference for the internal calculations that are done by the pH amplifier or transmitter that interprets the reading taken by the pH probe. As the reference voltage vs actual pH for a mature probe changes, the known buffer solution provides a benchmark for the calculation. Each pH instrumentation manufacturer will have a slightly different method for performing a calibration, but in general, the system will have you step through the buffer solutions while an automated routine makes note of the expected voltage vs calibration voltage at each step. The computation algorithm will use this drift information to re-scale the calculation to re-establish accuracy.
Read More
Topics:
water treatment issues,
water quality,
pH levels of water,
iron oxidation,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
ION Exchange Resin,
carbon dioxide,
gases,
RO system,
Aqua Farming
In the production and purification of water for industry
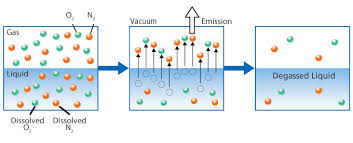
there are many types of different processes available to remove harmful minerals and gases from the water stream but the most effective process and most cost-effective from both a capital investment and operational cost is a “Forced Draft Degasification System” (Degasifier).
Degasification is used in a wide range of water processes for industrial and municipal applications which extend from the production of chemicals to the production of semiconductors and in all applications the need to remove contaminants from the water and dissolved gases is key to achieving the end results needed in the industrial water process. Water from the ground often contains elements such as calcium carbonate, manganese, iron, salts, hydrogen sulfide, and sulfur just to name a few of the basic contaminants and these naturally occurring elements can cause serious damage and consequences to process equipment such as boiler systems, piping, membranes, and cation and anion exchange resins used in the demineralization process.
Calcium carbonate can dissolve in water under certain pH ranges forming carbonic acid and releasing carbon dioxide (CO2) gases. These gases are not only very corrosive to equipment like boiler feed systems and boiler tubes but also attack the actual resin beds found in cation and anion softening and demineralization system causing an increase in regeneration and chemical consumption and resin bed replacement.
By incorporating a Force Draft Degasification system you can remove dissolved gasses
like CO2 and hydrogen sulfide (H2S) to as low as 99.999% and improve the cation and anion system performance, extend the resin bed life, and lower the operating cost of the water treatment process.
Quite often Forced Draft Degasification is utilized “post” treatment to also remove newly formed dissolved gases prior to entering the boiler feed system to prevent corrosion damage within the tubes and feed system and pumps. These gases are easily removed with the forced draft degasifier at a much lower cost than chemical additives or liquid cell degasification that requires higher capital cost and much higher operating cost.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
iron oxidation,
water treatment,
water distribution system,
aluminum,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
calcium carbonate,
media packing,
pH levels,
Langilier index (LSI),
Decarbonation,
ION Exchange Resin,
dissolved gases,
feed water,
De-Aeration,
wastewater,
carbon dioxide,
decarbonator,
degasifier,
carbonic acid,
H2S Degasifier
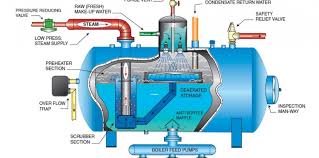
In the United States manufacturing industry, an astonishing 400 million gallons of water per day (MGD) is consumed to generate steam.
Out of this amount, approximately 60 MGD is sent to blow-down drains, while another 300 MGD is used for direct injection of steam. The common denominator in all of these processes is the need for purified and treated water. Without proper treatment, manufacturers would face frequent shutdowns and increased capital expenditure, significantly impacting their cost of goods. One effective method of water treatment to protect boilers is through degasification and deaeration.
Degasification towers play a crucial role in removing harmful gases such as hydrogen sulfide (H2S), carbon dioxide (CO2), and often dissolved oxygen (DO). The elimination of these corrosive gases is vital for enhancing the lifespan and efficiency of boiler systems. If these gases are allowed to remain in the boiler feed water, particularly carbon dioxide (CO2), it can lead to disastrous consequences, including higher operating costs and reduced system longevity. Carbon dioxide (CO2) can convert into carbonic acid, creating a corrosive environment for the boiler and other critical components. In cases where an ion exchange process is implemented prior to the boiler, the presence of carbon dioxide (CO2) can drastically increase regeneration costs as the resins are consumed. By removing carbon dioxide (CO2), the life of the resin is extended, and the pH of the water is elevated, reducing the need for additional chemicals and further lowering operating costs.
Read More
Topics:
water treatment issues,
degasification,
iron oxidation,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
Decarbonation,
ION Exchange Resin,
feed water,
De-Aeration,
steam generation,
steam generating boilers,
carbon dioxide,
steam,
decarbonator,
boiler system,
degasifier,
gases,
RO membrane,
carbonic acid,
RO system,
H2S Degasifier,
Boiler feed water
Avoid problems with calcium chlorite and corrosive gasses with your odor control scrubber.
When planning or designing an odor control system, one should pay close attention to several key variables that can cause havoc on a chemical odor control scrubber when trying to treat hydrogen sulfide or ammonia gases. The need for odor control occurs in many different forms. It is essential to understand the process that is creating the odorous or corrosive gas and the need for odor control & air emissions treatment.
First, begin to identify
all the potential obstacles that may creep up later after the chemical odor or corrosive gas control system goes online, like acid or caustic consumption. For example, chemical odor control systems designed for water treatment for the municipal industry are typically needed and attached to a degasification or decarbonation process, often needed to treat hydrogen sulfide (H2S). However, designers often may not pay close enough attention to the type of water process available for “make-up” water for the chemical scrubber. The addition of caustic can create scaling or fouling. This unknown variable of the makeup water quality can lead to a complete tower shutdown if the chemical scrubber distribution and media bed scales or fouls. The most commonly used chemicals for a hydrogen sulfide (H2S) scrubber are either chlorine in the form of sodium hypochlorite or caustic in the form of caustic soda. Both of these chemicals are common to a water treatment facility and are already in place to adjust and control pH.
The makeup water plays a significant role in the operation of a chemical scrubber.
When water containing high hardness levels is used as the source for the makeup water, your chemical scrubber can become fouled, and scaling can occur in a matter of hours, depending on the alkalinity and salts within the water. Solidification can occur from the scaling when combining sodium hypochlorite and raw feed water at specific pH ranges and these ranges are usually the range needed to achieve peak performance. Calcium chloride will form, and your chemical odor control scrubber will become a solid chunk of calcium chlorite making, making the ability for water or air to pass freely through the media packing next to impossible. No matter what type of media packing is utilized in the odor control or gas scrubber, it can foul and scale if the water chemistry is incorrect. Trust me when I say “been there and done that”! I have seen operators who have allowed a chemical scrubber to become out of balance with pH control and completely solidify the tower column to the degree that neither air nor water passage is possible. The problem can still occur with ammonia scrubbers but are different with different sets of parameters.
Read More
Topics:
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
calcium carbonate,
media packing,
pH levels,
Alkalinity,
Langilier index (LSI),
scaling,
chlorine,
caustic,
ION Exchange Resin,
Safe drinking water,
dissolved gases,
De-Aeration,
carbon dioxide,
oxygen,
degasifier,
gases,
H2S Degasifier,
calcium chlorite
Many types of water treatment systems depend on some type of media to provide the best performance required as it relates to water treatment and waste water treatment. For use in reverse osmosis there is a reliance on membranes which act as filters to separate the solids from the water. For ion exchange there are “resins” whether AION or CATION the resins works to treat hard and corrosive water. Degasification and decarbonation towers both require an internal media and sometimes this is referred to as “Random Packing” or “Loose Fill Media” and in this process the media acts like a traffic cop directing traffic.
In this case it directs the water on its way down and through a towers internals where it is constantly reshaping the water droplets over and over again forcing gas molecules to come to the surface edge of the water where they are removed. Carbon filters also require a media which is of course “Carbon”. The carbon media acts like a sponge absorbing the contaminants that you wish to remove from the water until it is saturated and must be replaced or regenerated. Even sand filters or pressure filters require a media.
Read More
Topics:
degasification,
water treatment,
water plant,
media packing,
Decarbonation,
ION Exchange Resin,
feed water,
wastewater,
decarbonator,
gases,
RO membrane