In modern industrial water treatment, advancements in technology and processes have revolutionized the way contaminants are removed from water.
This blog explores the integration of NSF/ANSI 61 certified systems, artificial intelligence in water treatment, and cutting-edge processes such as decarbonation and degasification. We'll also discuss the key differences between forced draft and induced draft degasification towers, helping you make informed decisions while designing your Industrial Water Treatment System.
-
NSF/ANSI 61-Certified Water Treatment Systems: To ensure the safety and quality of water treatment equipment, NSF/ANSI 61 certification has become a crucial standard. This certification verifies that materials and components used in water treatment systems comply with health and safety requirements. When selecting a water treatment solution, opting for NSF/ANSI 61 certified systems guarantees peace of mind and adherence to the highest industry standards.
-
Harnessing Artificial Intelligence in Water Treatment: Artificial intelligence (AI) has penetrated various industries, and water treatment is no exception. Integrating AI into water treatment processes allows for more efficient and optimized operations. AI-driven systems can monitor water quality in real-time, predict system failures, optimize chemical dosing, and reduce energy consumption. By leveraging AI technologies, water treatment facilities can enhance their overall performance and streamline resource utilization.
-
Decarbonation and Degasification Systems: Decarbonation and degasification are essential processes in industrial water treatment, particularly in pH levels in water and the ability to control removing the contaminants. These processes target the removal of carbon dioxide (CO2) and other dissolved gases from water to improve its quality. Two key systems used for this purpose are the decarbonator and aeration system.
Read More
Topics:
degasification,
advanced treatment solutions,
biological scrubber,
NSF/ANSI 61,
Chemical Odor,
Decarbonation,
Safe drinking water,
De-Aeration,
decarbonator,
degasifier,
degassed water,
ansi61,
nsf/ansi61,
Deagasification,
decarbonation of water,
DeLoach Industries, Inc.,
Drinking Water,
Industrial Odor Control,
DeLoach Industries,
contaminants,
process system,
safe drinking water act,
drinking water standards,
environmental safety,
air emissions,
Forced Draft,
Induced Draft
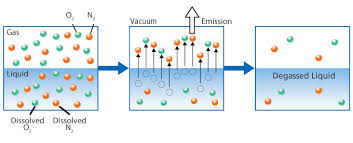
Requires an application commonly referred to as either “Degasification” or "Decarbonation" and it requires the use of a piece of water treatment equipment called either a “degasifier” or a “decarbonator”.
Both of these are similar in nature and are designed for Carbon Dioxide (CO2) removal from the incoming water. A properly designed decarbonator can remove 99.99% of the free carbon dioxide gas that is present in the water stream. One of the primary reasons for utilizing a decarbonator or degasifier for the removal of carbon dioxide gas is the raise the pH of the water without the need to add caustic. resulting in high-purity water.
The other reason is the remove the CO2 prior to treating the water with Ion Exchange which utilizes Anion or Cation resins to reduce the regeneration cycles for the resin beds. High concentrations of CO2 consume the ion charge within the resins and require more frequent regeneration cycles. The difference between anion and cation resins is that one is positively charged (anion) and the other is negatively charged (cation), cation resins, attract positive ions with their negative charge.
The term decarbonation describes the process of the removal of suspended gas or the conversion of carbonic acids into free Carbon Dioxide. Carbonic Acid (H2CO3) is stable at normal ambient anhydrous conditions. However, Carbonic Acid decomposes when not stable and in the presence of any water molecules to form carbon dioxide (CO2). The Carbonic acid breaks down when present in water and it is converted to a gas based upon certain conditions. It is common to have CO2 present in water requiring a decarbonation process when utilizing certain types of water filtration such as membrane filtration with reverse osmosis or it can be present when the need to adjust pH is required. When removing (CO2) the process is often referred to as “Decarbonation”, when removing (H2S) Hydrogen Sulfide the process is often referred to as “Degasification”.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier
The Basics of Water Decarbonation
and the removal of carbon dioxide (CO2). The need to remove (CO2) is essential in most Aquaculture, Municipal, Industrial, and Food & Beverage Processes To understand you must familiarize yourself with Henry’s Law.
Henry's Law defines the method and proportional relationship between the amount of a gas in a solution in relation to the gas's partial pressure in the atmosphere. Often you will see and hear various terms like degasification, decarbonation, aeration, and even air stripping when discussing the removal of dissolved gases and other convertible elements from water. Understanding the impacts that Carbon Dioxide (CO2) can have on both equipment and aquatic life provides the basic reasons why the need to decarbonate water, exists. Carbon Dioxide (CO2) can exist naturally in the raw water supply or be the result of ph control and balance. In either case, the process called Decarbonation or Degasification provides the most cost-effective and efficient manner to reduce or tally remove (CO2) from the water. In addition to Carbon Dioxide (CO2), water can contain a variety of other contaminants that may impact the removal efficiency of the Carbon Dioxide. A variety of elements as well as dissolved gases such as oxygen, nitrogen, and carbon dioxide (CO2). A full analytical review of the water chemistry is required to properly design and size the “Water Treatment” process.
Breaking the bonds in water releases a dissolved gas
such as carbon dioxide (CO2) you must change the conditions of the vapor pressure surrounding the gas and allow the gas to be removed. There are many variables to consider when designing or calculating the “means and methods” of the removal of carbon dioxide (CO2). When I refer to the means and methods. I am referring to the design of a decarbonator and its components. The means equals the size and type (Hydraulic load) of the decarbonator and the “method” equals the additional variables such as the cubic foot of airflow (CFM) and “Ratio” of the air to water to accomplish the proportional condition needed to remove the carbon dioxide (CO2).
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
removal of CO2 from water
Water turbidity refers to how transparent or translucent the water is when examining or testing it for any use.
Water turbidity can impact food and beverage, municipal, industrial, and aquaculture operations. Turbidity is caused by suspended or dissolved particles in the water that scatter light which causes the water to appear cloudy or even murky.
Different particles can cause turbidity, including sediments such as silts and clay, fine inorganic or organic matter, algae or soluble colored organic compounds, and microscopic organisms. Turbidity is measured in a value referred to as NTU, which means Nephelometric Turbidity Unit. The EPA requires a turbidity level no higher than 0.3 NTU in the USA, and if a member of the partnership of safe drinking water, then the level must not exceed 0.1 NTU.
High turbidity can create habitats for other harmful elements, such as bacteria or metals, that can accumulate onto the particles. This increases the health risk for a potable water system. In aquaculture operations, increased turbidity from silts and sediments can harm and harm marine life, so it must be removed to safe levels. For the food and beverage industry, the impact of high turbidity can be both a safety concern and a visual and noticeable quality concern because if the turbidity is high, it can alter the physical look of the final product, for example, a distillery.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
Safe drinking water,
De-Aeration,
decarbonator,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture,
Deagasification,
particulate matter,
filters,
Sand filters,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
turbidity
Optimizing Water Quality and Enhancing Efficiency.
To enhance and balance the water quality in aquaculture and pisciculture operations, the industry is recognizing the benefits of utilizing a type of water treatment called “Forced Draft Degasification” to remove CO2 (Carbon Dioxide) and H2S (Hydrogen Sulfide) gases and oxidize other elements such as Iron or Magnesium.
Removing these elements from the water process improves the quality of the water and aids in the balancing of the pH without the need for additional chemicals. This also reduces the risk of unnecessary and preventable loss of life to your aqua farming project. Keeping the ph at the proper level will enable a good healthy environment and will prevent further problems that may occur due to the ph is not kept in a stable state.
Read More
Topics:
De-Aeration,
carbon dioxide,
decarbonator,
degasifier,
gases,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
In the production and purification of water for industry
there are many types of different processes available to remove harmful minerals and gases from the water stream but the most effective process and most cost-effective from both a capital investment and operational cost is a “Forced Draft Degasification System” (Degasifier).
Degasification is used in a wide range of water processes for industrial and municipal applications which extend from the production of chemicals to the production of semiconductors and in all applications the need to remove contaminants from the water and dissolved gases is key to achieving the end results needed in the industrial water process. Water from the ground often contains elements such as calcium carbonate, manganese, iron, salts, hydrogen sulfide, and sulfur just to name a few of the basic contaminants and these naturally occurring elements can cause serious damage and consequences to process equipment such as boiler systems, piping, membranes, and cation and anion exchange resins used in the demineralization process.
Calcium carbonate can dissolve in water under certain pH ranges forming carbonic acid and releasing carbon dioxide (CO2) gases. These gases are not only very corrosive to equipment like boiler feed systems and boiler tubes but also attack the actual resin beds found in cation and anion softening and demineralization system causing an increase in regeneration and chemical consumption and resin bed replacement.
By incorporating a Force Draft Degasification system you can remove dissolved gasses
like CO2 and hydrogen sulfide (H2S) to as low as 99.999% and improve the cation and anion system performance, extend the resin bed life, and lower the operating cost of the water treatment process.
Quite often Forced Draft Degasification is utilized “post” treatment to also remove newly formed dissolved gases prior to entering the boiler feed system to prevent corrosion damage within the tubes and feed system and pumps. These gases are easily removed with the forced draft degasifier at a much lower cost than chemical additives or liquid cell degasification that requires higher capital cost and much higher operating cost.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
iron oxidation,
water treatment,
water distribution system,
aluminum,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
calcium carbonate,
media packing,
pH levels,
Langilier index (LSI),
Decarbonation,
ION Exchange Resin,
dissolved gases,
feed water,
De-Aeration,
wastewater,
carbon dioxide,
decarbonator,
degasifier,
carbonic acid,
H2S Degasifier
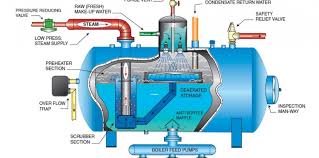
In the United States manufacturing industry, an astonishing 400 million gallons of water per day (MGD) is consumed to generate steam.
Out of this amount, approximately 60 MGD is sent to blow-down drains, while another 300 MGD is used for direct injection of steam. The common denominator in all of these processes is the need for purified and treated water. Without proper treatment, manufacturers would face frequent shutdowns and increased capital expenditure, significantly impacting their cost of goods. One effective method of water treatment to protect boilers is through degasification and deaeration.
Degasification towers play a crucial role in removing harmful gases such as hydrogen sulfide (H2S), carbon dioxide (CO2), and often dissolved oxygen (DO). The elimination of these corrosive gases is vital for enhancing the lifespan and efficiency of boiler systems. If these gases are allowed to remain in the boiler feed water, particularly carbon dioxide (CO2), it can lead to disastrous consequences, including higher operating costs and reduced system longevity. Carbon dioxide (CO2) can convert into carbonic acid, creating a corrosive environment for the boiler and other critical components. In cases where an ion exchange process is implemented prior to the boiler, the presence of carbon dioxide (CO2) can drastically increase regeneration costs as the resins are consumed. By removing carbon dioxide (CO2), the life of the resin is extended, and the pH of the water is elevated, reducing the need for additional chemicals and further lowering operating costs.
Read More
Topics:
water treatment issues,
degasification,
iron oxidation,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
Decarbonation,
ION Exchange Resin,
feed water,
De-Aeration,
steam generation,
steam generating boilers,
carbon dioxide,
steam,
decarbonator,
boiler system,
degasifier,
gases,
RO membrane,
carbonic acid,
RO system,
H2S Degasifier,
Boiler feed water
Avoid problems with calcium chlorite and corrosive gasses with your odor control scrubber.
When planning or designing an odor control system, one should pay close attention to several key variables that can cause havoc on a chemical odor control scrubber when trying to treat hydrogen sulfide or ammonia gases. The need for odor control occurs in many different forms. It is essential to understand the process that is creating the odorous or corrosive gas and the need for odor control & air emissions treatment.
First, begin to identify
all the potential obstacles that may creep up later after the chemical odor or corrosive gas control system goes online, like acid or caustic consumption. For example, chemical odor control systems designed for water treatment for the municipal industry are typically needed and attached to a degasification or decarbonation process, often needed to treat hydrogen sulfide (H2S). However, designers often may not pay close enough attention to the type of water process available for “make-up” water for the chemical scrubber. The addition of caustic can create scaling or fouling. This unknown variable of the makeup water quality can lead to a complete tower shutdown if the chemical scrubber distribution and media bed scales or fouls. The most commonly used chemicals for a hydrogen sulfide (H2S) scrubber are either chlorine in the form of sodium hypochlorite or caustic in the form of caustic soda. Both of these chemicals are common to a water treatment facility and are already in place to adjust and control pH.
The makeup water plays a significant role in the operation of a chemical scrubber.
When water containing high hardness levels is used as the source for the makeup water, your chemical scrubber can become fouled, and scaling can occur in a matter of hours, depending on the alkalinity and salts within the water. Solidification can occur from the scaling when combining sodium hypochlorite and raw feed water at specific pH ranges and these ranges are usually the range needed to achieve peak performance. Calcium chloride will form, and your chemical odor control scrubber will become a solid chunk of calcium chlorite making, making the ability for water or air to pass freely through the media packing next to impossible. No matter what type of media packing is utilized in the odor control or gas scrubber, it can foul and scale if the water chemistry is incorrect. Trust me when I say “been there and done that”! I have seen operators who have allowed a chemical scrubber to become out of balance with pH control and completely solidify the tower column to the degree that neither air nor water passage is possible. The problem can still occur with ammonia scrubbers but are different with different sets of parameters.
Read More
Topics:
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
calcium carbonate,
media packing,
pH levels,
Alkalinity,
Langilier index (LSI),
scaling,
chlorine,
caustic,
ION Exchange Resin,
Safe drinking water,
dissolved gases,
De-Aeration,
carbon dioxide,
oxygen,
degasifier,
gases,
H2S Degasifier,
calcium chlorite
Optimizing Steam Process Water Systems with Degasification Towers
Steam process water systems are integral to various industrial operations, where water is heated and converted into steam. However, ensuring the efficiency and longevity of these systems requires a comprehensive understanding of water chemistry and the implementation of proper treatment methods. In particular, the removal of dissolved gases, such as hydrogen sulfide (H2S), carbon dioxide (CO2), and dissolved oxygen (O2), is crucial. This blog post will delve into the significance of degasification towers in steam process water systems, emphasizing their role in preventing corrosion, enhancing equipment performance, and maintaining water quality in your water and wastewater systems.
The Importance of Removing Dissolved Gases
Dissolved gases in steam process water systems can have detrimental effects on boilers and other critical components. Allowing gases like carbon dioxide (CO2) to remain in the water leads to the formation of carbonic acid, creating a corrosive environment. This corrosion can damage the boiler and reduce its lifespan. Additionally, dissolved gases can impair the efficiency of the system, affecting heat transfer and leading to reduced performance.
Read More
Topics:
De-Aeration,
carbon dioxide,
oxygen,
steam,
decarbonator,
degasifier,
carbonic acid
Do you think all distribution systems are made equal?
if you do you may be surprised that there is a lot of variation in manufacturing protocols for aerators, degasifiers, and decarbonators. Aerators are often found in use at Industrial Water Treatment and municipal water treatment facilities around the globe.
For water treatment, you may be surprised to learn that one of the key items that separate different types of aerators and decarbonators for water treatment is the type of distribution system it utilizes. To improve Carbon Dioxide (CO2) or Hydrogen Sulfide (H2S) removal you need to select the best distribution system for the tower and make sure it's maintained. Now, there are many types of aerators in general and the term is used broadly. From floating pond aerators to wastewater aerators, to vertical tower aerators, decarbonators, and degasifiers for industrial water treatment aerators. We will focus on vertical tower aerators for industrial water treatment. All types of Aerators and even degasifiers and even decarbonators and Odor Control Scrubbers require some type of distribution system to begin the process of gas transfer and to remove Hydrogen Sulfide (H2S) from water or Carbon Dioxide (CO2). It is important to evenly distribute the water or chemical solution across the media bed.
There are several types of distribution systems available and the three most common ones you will see on the marketplace are the “Tray” type, Weir, or the header lateral utilizing gas release “Nozzles”.
The selection of what type of distribution system is typically driven by the marketing side of who is selling you the tower. But in terms of real performance a distribution system utilizing a nozzle system will outperform a tray-type distributor. All packed towers are designed utilizing Henry’s Law Constant” theory of chemistry and what all towers rely upon is some type of method to break the surface tension of the water and expose the molecules of gases so that they either can escape or can be introduced to a reaction agent.
When towers are designed it is important to properly hydraulically load the top of the media bed. This is considered " Degasification Basics". This is important for many reasons and we will address these points in future updates. When using a properly designed nozzle distribution system such as a DeLoach Industries header lateral system then you get the benefit of both proper hydraulic load across the bed and you also gain anywhere from 4-10% removal efficiency depending upon the application. When looking at a chemical scrubber versus a biological scrubber you will notice they too have very different distribution systems. DeLoach Industries, Inc. has learned over its 60 years in business how to maximize gas transfer release. If designed and built properly the gas release process or interaction process (if designing a scrubber) has already begun “before” it enters the media bed.
Read More
Topics:
water treatment issues,
aeration,
Decarbonation,
De-Aeration,
decarbonator,
degasifier