In a world increasingly impacted by climate change, industrialization, and population growth, ensuring access to clean, safe water has never been more critical. At the forefront of this global challenge is the advancement of water filtration technologies—systems designed not only to purify water but to do so with greater efficiency, lower environmental impact, and higher performance. Water treatment professionals are now turning to cutting-edge solutions that revolutionize how we clean, recycle, and reuse one of our planet’s most vital resources.
Read More
Topics:
water quality,
water treatment,
RO membrane,
DeLoach Industries, Inc.,
reverse osmosis,
membrane technology,
pfas removal,
nanofiltration,
filtration technologies
If you’ve been reading the news lately, you know nanoparticles are not so great. In everything from cosmetics to water filters, nanoparticles have been shown to cause various health problems. But what exactly are nanoparticles, and how can you protect yourself from their harmful effects? Let’s answer these questions and more with this quick guide on removing nanoparticles from your drinking water.
Read More
Topics:
water treatment issues,
water quality,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
safety,
Safe drinking water,
Global,
distillation,
RO membrane,
RO system,
particulate matter,
filters,
municipal water systems,
residential well water systems,
DeLoach Industries, Inc.,
Drinking Water,
Clean Water,
Water Test,
Water Test Kit,
DeLoach Industries,
technology,
minerals,
temperature,
nanoparticles,
Cosmetics,
Nano,
make-up,
organ function,
contaminants,
pressure filters,
reverse osmosis,
carbon filters,
UV filters,
activated carbon
DeLoach Industries made history in 1977 at the City of Cape Coral Florida water treatment plant with its large scale “degasification towers” connected to what was to become the first municipal water treatment facility in the United States to deploy the use of reverse osmosis on a large-scale production municipal treatment plant.
The Cape Coral water treatment plant for came online in 1977 and produced 3 million gallons of water per day (GPD) or 11.35 liters of purified and treated water utilizing the “reverse osmosis” process. By 1985 the plant had expanded as it kept up with growth to produce 15 million gallons per day making it at the time the world’s largest “reverse osmosis” water treatment plant facility.
Read More
Topics:
water quality,
pH levels of water,
water treatment,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
scaling,
chlorine,
caustic,
Decarbonation,
wastewater,
carbon dioxide,
degasifier,
RO membrane,
RO system,
H2S Degasifier
In the United States manufacturing industry, an astonishing 400 million gallons of water per day (MGD) is consumed to generate steam.
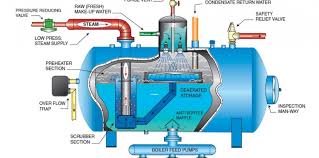
Out of this amount, approximately 60 MGD is sent to blow-down drains, while another 300 MGD is used for direct injection of steam. The common denominator in all of these processes is the need for purified and treated water. Without proper treatment, manufacturers would face frequent shutdowns and increased capital expenditure, significantly impacting their cost of goods. One effective method of water treatment to protect boilers is through degasification and deaeration.
Degasification towers play a crucial role in removing harmful gases such as hydrogen sulfide (H2S), carbon dioxide (CO2), and often dissolved oxygen (DO). The elimination of these corrosive gases is vital for enhancing the lifespan and efficiency of boiler systems. If these gases are allowed to remain in the boiler feed water, particularly carbon dioxide (CO2), it can lead to disastrous consequences, including higher operating costs and reduced system longevity. Carbon dioxide (CO2) can convert into carbonic acid, creating a corrosive environment for the boiler and other critical components. In cases where an ion exchange process is implemented prior to the boiler, the presence of carbon dioxide (CO2) can drastically increase regeneration costs as the resins are consumed. By removing carbon dioxide (CO2), the life of the resin is extended, and the pH of the water is elevated, reducing the need for additional chemicals and further lowering operating costs.
Read More
Topics:
water treatment issues,
degasification,
iron oxidation,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
Decarbonation,
ION Exchange Resin,
feed water,
De-Aeration,
steam generation,
steam generating boilers,
carbon dioxide,
steam,
decarbonator,
boiler system,
degasifier,
gases,
RO membrane,
carbonic acid,
RO system,
H2S Degasifier,
Boiler feed water
The water treatment industry has developed and evolved over the years to continue to find new ways to produce degassed water,
Read More
Topics:
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
water plant,
safety,
hydrogen sulfide (H2S),
Chemical Odor,
media packing,
pH levels,
Decarbonation,
dissolved gases,
wastewater,
Global,
carbon dioxide,
decarbonator,
degasifier,
gases,
RO membrane,
H2S Degasifier,
degassed water
Many types of water treatment systems depend on some type of media to provide the best performance required as it relates to water treatment and waste water treatment. For use in reverse osmosis there is a reliance on membranes which act as filters to separate the solids from the water. For ion exchange there are “resins” whether AION or CATION the resins works to treat hard and corrosive water. Degasification and decarbonation towers both require an internal media and sometimes this is referred to as “Random Packing” or “Loose Fill Media” and in this process the media acts like a traffic cop directing traffic.
In this case it directs the water on its way down and through a towers internals where it is constantly reshaping the water droplets over and over again forcing gas molecules to come to the surface edge of the water where they are removed. Carbon filters also require a media which is of course “Carbon”. The carbon media acts like a sponge absorbing the contaminants that you wish to remove from the water until it is saturated and must be replaced or regenerated. Even sand filters or pressure filters require a media.
Read More
Topics:
degasification,
water treatment,
water plant,
media packing,
Decarbonation,
ION Exchange Resin,
feed water,
wastewater,
decarbonator,
gases,
RO membrane
Protecting Your Pharmaceutical Water: Ensuring Quality and Efficiency in Water Treatment
In the pharmaceutical industry, the removal of dissolved gases from water is a critical step in the water treatment process. However, it is essential to select the appropriate method of removing these gases, as the wrong choice can have detrimental effects on vital process water equipment such as steam boilers and distillation columns. Failure to address high levels of carbon dioxide (CO2) in the water can lead to the formation of carbonic acid, which corrodes and damages both the steam boiler tubes and distillation columns. To mitigate these risks, the implementation of a degasification tower or "Degasifier" is crucial, as it effectively removes dissolved gases like hydrogen sulfide (H2S) and carbon dioxide (CO2) to acceptable levels below 7 parts per billion (ppb).
Utilizing a degasification tower offers a cost-effective solution to reduce and eliminate gases in the water stream. In comparison, alternative methods such as reverse osmosis (RO) membranes require additional steps, including pH adjustment, to achieve similar results. The conversion of carbon dioxide (CO2) into carbonates can result in increased membrane fouling and elevated capital costs for the RO system. By implementing a degasification system, businesses can achieve optimal performance, minimize membrane fouling, and benefit from cost savings in both capital and operational expenses.
Read More
Topics:
degasification,
water treatment,
hydrogen sulfide (H2S),
dissolved gases,
pharmaceutical water,
carbon dioxide,
degasifier,
gases,
RO membrane,
carbonic acid,
RO system