The type of Odor Control Wet Scrubber selected for the treatment and neutralization of Ammonia (NH3) gases depends on several variables, including the type and source of the ammonia gas and whether or not it is “Free” ammonia and or unionized.
Ammonia is a very miscible and stable molecule with solid hydrogen bonds, making it very soluble in water and difficult to treat without using a properly designed and sized ammonia scrubber. The concentrations, air flow rates, temperature of the gas stream, and chemical reagents being utilized, such as caustic to remove and then treat the ammonia, all play a significant role in the removal efficiency of the ammonia gas scrubber system. Unlike other types of “odor control scrubbers,” an ammonia scrubber is much more sensitive to variables such as the gas stream temperature because of the solubility of ammonia.
Ammonia is produced from nitrogen and hydrogen
the process is called the Haber Process by combining nitrogen with air and adding pressure, you can make ammonia.
It takes about 200 atmospheres of pressure, and the process varies from refinery to refinery. Still, on average, you can only make approximately 15% of ammonia during each pass which takes multiple passes to achieve the 15%. The reaction to make ammonia is exothermic when produced in a refining process.
However, ammonia is also formed in nature in smaller quantities. Most ammonia (90%) is utilized for fertilizer production, but ammonia can be found in food, pharmaceutical products, and cleaning supplies. When ammonia gas is released into the air, it has a very noxious and pungent odor that can be dangerous to inhale, so often, odor control scrubbers are required to capture and treat the ammonia gas.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
pH levels,
Decarbonation,
dissolved gases,
wastewater,
degasifier,
gases,
H2S Degasifier,
Ammonia
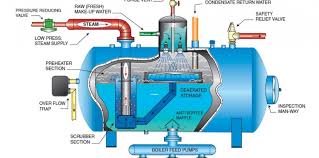
In the United States manufacturing industry, an astonishing 400 million gallons of water per day (MGD) is consumed to generate steam.
Out of this amount, approximately 60 MGD is sent to blow-down drains, while another 300 MGD is used for direct injection of steam. The common denominator in all of these processes is the need for purified and treated water. Without proper treatment, manufacturers would face frequent shutdowns and increased capital expenditure, significantly impacting their cost of goods. One effective method of water treatment to protect boilers is through degasification and deaeration.
Degasification towers play a crucial role in removing harmful gases such as hydrogen sulfide (H2S), carbon dioxide (CO2), and often dissolved oxygen (DO). The elimination of these corrosive gases is vital for enhancing the lifespan and efficiency of boiler systems. If these gases are allowed to remain in the boiler feed water, particularly carbon dioxide (CO2), it can lead to disastrous consequences, including higher operating costs and reduced system longevity. Carbon dioxide (CO2) can convert into carbonic acid, creating a corrosive environment for the boiler and other critical components. In cases where an ion exchange process is implemented prior to the boiler, the presence of carbon dioxide (CO2) can drastically increase regeneration costs as the resins are consumed. By removing carbon dioxide (CO2), the life of the resin is extended, and the pH of the water is elevated, reducing the need for additional chemicals and further lowering operating costs.
Read More
Topics:
water treatment issues,
degasification,
iron oxidation,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
Decarbonation,
ION Exchange Resin,
feed water,
De-Aeration,
steam generation,
steam generating boilers,
carbon dioxide,
steam,
decarbonator,
boiler system,
degasifier,
gases,
RO membrane,
carbonic acid,
RO system,
H2S Degasifier,
Boiler feed water
The water treatment industry has developed and evolved over the years to continue to find new ways to produce degassed water,
Read More
Topics:
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
water plant,
safety,
hydrogen sulfide (H2S),
Chemical Odor,
media packing,
pH levels,
Decarbonation,
dissolved gases,
wastewater,
Global,
carbon dioxide,
decarbonator,
degasifier,
gases,
RO membrane,
H2S Degasifier,
degassed water
Many types of water treatment systems depend on some type of media to provide the best performance required as it relates to water treatment and waste water treatment. For use in reverse osmosis there is a reliance on membranes which act as filters to separate the solids from the water. For ion exchange there are “resins” whether AION or CATION the resins works to treat hard and corrosive water. Degasification and decarbonation towers both require an internal media and sometimes this is referred to as “Random Packing” or “Loose Fill Media” and in this process the media acts like a traffic cop directing traffic.
In this case it directs the water on its way down and through a towers internals where it is constantly reshaping the water droplets over and over again forcing gas molecules to come to the surface edge of the water where they are removed. Carbon filters also require a media which is of course “Carbon”. The carbon media acts like a sponge absorbing the contaminants that you wish to remove from the water until it is saturated and must be replaced or regenerated. Even sand filters or pressure filters require a media.
Read More
Topics:
degasification,
water treatment,
water plant,
media packing,
Decarbonation,
ION Exchange Resin,
feed water,
wastewater,
decarbonator,
gases,
RO membrane
One of the largest consumers of energy in the US is water and wastewater treatment plants.
Because of the need for large horsepower pumps and blowers, a municipal water and wastewater treatment plant consumes a tremendous amount of kilowatt hours of electricity. The energy cost is factored into the “cost of production” of water or wastewater treatment, and the “rate base” charge is increased accordingly to the consumer.
Does Renewable Power Work in a Water Treatment Plant?
Because solar energy is “space intensive,” you do not see a lot of solar power being deployed across the USA at water treatment plants. In our opinion, this is a mistake, and most likely, the decision was made back when solar power output was much lower. With the increased efficiency of solar panels and decreased production cost, it makes tremendous sense to revisit the use of Solar energy to offset the operational cost of a water treatment plant or wastewater treatment plant operation.
Providing solar energy for specific pieces of process equipment is also a viable option when you consider deploying solar energy. For example, operating a Degasification tower or Decarbonator utilizing 10 350-watt solar panels will generate 3500 watts during peak daylight hours and enough to offset the cost of smaller horsepower blower motors. If the solar panels are configured as a canopy, they can also provide a nice shade or protective barrier above the piece of equipment if installed outdoors, as most packed column towers are located outside.
What about other forms of renewable energy? Do they work?
At water treatment or wastewater treatment facilities. Co-generation use has been around for many years at Wastewater plant facilities wastewater treatment plants. A cogeneration unit is a combination “Generator” to produce power and a “Thermal” energy source to produce heated water. The water can be used domestically or can be used to produce chilled water with the help of a Chiller system. The wastewater treatment plant provides a critical component by producing gases such as “Methane,” which can be used as a cogeneration unit fuel source. Water treatment plants do not produce methane or other combustible forms of gases like a cogeneration plant would produce, so you normally do not see Cogeneration system units deployed at a Water treatment facility.
Read More
Topics:
degasification,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
Decarbonation,
wastewater,
Recycling,
Global,
steam generation,
steam
Do you think all distribution systems are made equal?
if you do you may be surprised that there is a lot of variation in manufacturing protocols for aerators, degasifiers, and decarbonators. Aerators are often found in use at Industrial Water Treatment and municipal water treatment facilities around the globe.
For water treatment, you may be surprised to learn that one of the key items that separate different types of aerators and decarbonators for water treatment is the type of distribution system it utilizes. To improve Carbon Dioxide (CO2) or Hydrogen Sulfide (H2S) removal you need to select the best distribution system for the tower and make sure it's maintained. Now, there are many types of aerators in general and the term is used broadly. From floating pond aerators to wastewater aerators, to vertical tower aerators, decarbonators, and degasifiers for industrial water treatment aerators. We will focus on vertical tower aerators for industrial water treatment. All types of Aerators and even degasifiers and even decarbonators and Odor Control Scrubbers require some type of distribution system to begin the process of gas transfer and to remove Hydrogen Sulfide (H2S) from water or Carbon Dioxide (CO2). It is important to evenly distribute the water or chemical solution across the media bed.
There are several types of distribution systems available and the three most common ones you will see on the marketplace are the “Tray” type, Weir, or the header lateral utilizing gas release “Nozzles”.
The selection of what type of distribution system is typically driven by the marketing side of who is selling you the tower. But in terms of real performance a distribution system utilizing a nozzle system will outperform a tray-type distributor. All packed towers are designed utilizing Henry’s Law Constant” theory of chemistry and what all towers rely upon is some type of method to break the surface tension of the water and expose the molecules of gases so that they either can escape or can be introduced to a reaction agent.
When towers are designed it is important to properly hydraulically load the top of the media bed. This is considered " Degasification Basics". This is important for many reasons and we will address these points in future updates. When using a properly designed nozzle distribution system such as a DeLoach Industries header lateral system then you get the benefit of both proper hydraulic load across the bed and you also gain anywhere from 4-10% removal efficiency depending upon the application. When looking at a chemical scrubber versus a biological scrubber you will notice they too have very different distribution systems. DeLoach Industries, Inc. has learned over its 60 years in business how to maximize gas transfer release. If designed and built properly the gas release process or interaction process (if designing a scrubber) has already begun “before” it enters the media bed.
Read More
Topics:
water treatment issues,
aeration,
Decarbonation,
De-Aeration,
decarbonator,
degasifier
Ammonia (AM) is a common water pollutant that significantly impacts the water process industry.
It is not just polluting water bodies but also aqua wells and humidifiers. Generally, AM is produced from human sweat and urine and created from synthetic ammonia in industrial processes.
Ammonia has three types of amines – primary, secondary, and tertiary – all are toxic for humans and aquatic life.
- Primary Amine has two carbon and one nitrogen atom, also called methylamine or CHNH2.
- Secondary Amine has two nitrogen atoms with no carbon atom between them, also called Dimethylamine or CH2(NH)CH3.
- Tertiary Amine has three nitrogen atoms with no carbon atoms between them; thus, it’s called Trimethylamine or CH3C(NH)CH3.
In natural conditions, primary Amide bacteria produce Amide under high-temperature conditions. In an aqueous solution and soil environments with high pH levels (>6).
Primary amide can form by the dehydrogenation of nitriles, such as acetonitrile, which are further oxidized to form acetic acid.
Primary amide form by alkaline hydrolysis of nitro compounds such as 2-nitrophenol.
Process systems often need to recognize when the Degasification or Decarbonation system is failing or underperforming.
Read More
Topics:
Decarbonation,
decarbonator,
degasifier,
Amine,
Ammonia,
Deagasification,
Filter Media,
distribution system,
blower motor,
process system,
frequent inspections
Understanding De-Aeration and Decarbonation in Water Treatment Systems
De-Aeration and decarbonation are two essential processes used to remove carbon dioxide (CO2) and dissolved oxygen (O2) from water streams, particularly in boiler-feed water systems. While both processes share the goal of eliminating CO2, they differ in their approach to removing oxygen. A De-Aeration system focuses on removing both CO2 and O2, while a decarbonation system primarily targets the removal of CO2. Let's delve deeper into these processes to understand their mechanisms and benefits.
In a De-Aeration system, steam is introduced at the bottom of the tower. The inlet feed water is heated to near saturation temperature, minimizing pressure drop and venting limits. This ensures optimal thermal operating efficiency of the tower. The steam acts as a carrier gas, stripping both CO2 and O2 from the water as it rises through the tower. The tower is equipped with an internal distribution system and media packing to enhance the removal of dissolved gases. By the time the water reaches the top of the tower, it has undergone significant de-aeration, resulting in reduced CO2 and O2 levels. This purified water is then ready for entry into the boiler, ensuring efficient and reliable steam generation.
Read More
Topics:
media packing,
Decarbonation,
De-Aeration,
carbon dioxide,
oxygen,
steam,
decarbonator
The importance of removing Carbon Dioxide in the water!
Carbon dioxide exists naturally in nature as free CO2 and can be found in many water sources from lakes, streams, or other surface water bodies. Carbon dioxide occurs naturally in small amounts (about 0.04 percent) in the Earth's atmosphere. Monitoring CO2 levels in your water can be done through test kits or monitoring systems. When monitoring CO2 levels, it is important to note the concentration at which the monitoring needs to occur. Industrial level ion exchange systems should be monitored at a concentration typically 15–20 times greater than required for drinking water quality. Ion exchange systems used for high purity water production should be monitored at a concentration typically 40–50 times greater than what is required for drinking water quality. Due to carbon dioxide’s abundance and its role as the primary driver of climate change, there are concerns about increasing concentrations of this gas in the atmosphere. To reduce the amount of carbon dioxide in the atmosphere, people can reduce the amount of carbon dioxide released during energy production by using renewable energy sources and energy efficiency. Carbon dioxide can be captured and stored underground with carbon sequestration technologies.
Read More
Topics:
degasification,
water treatment,
advanced treatment solutions,
Decarbonation,
ION Exchange Resin,
carbon dioxide,
CO2 in water,
excess co2,
hydrogen ion
Saving Steam with Degasification: Optimizing Water Treatment for Cost Efficiency and Enhanced Performance.
Read More
Topics:
degasification,
Decarbonation,
steam generation,
carbon dioxide,
steam,
decarbonator,
distillation