In modern industrial water treatment, advancements in technology and processes have revolutionized the way contaminants are removed from water.
This blog explores the integration of NSF/ANSI 61 certified systems, artificial intelligence in water treatment, and cutting-edge processes such as decarbonation and degasification. We'll also discuss the key differences between forced draft and induced draft degasification towers, helping you make informed decisions while designing your Industrial Water Treatment System.
-
NSF/ANSI 61-Certified Water Treatment Systems: To ensure the safety and quality of water treatment equipment, NSF/ANSI 61 certification has become a crucial standard. This certification verifies that materials and components used in water treatment systems comply with health and safety requirements. When selecting a water treatment solution, opting for NSF/ANSI 61 certified systems guarantees peace of mind and adherence to the highest industry standards.
-
Harnessing Artificial Intelligence in Water Treatment: Artificial intelligence (AI) has penetrated various industries, and water treatment is no exception. Integrating AI into water treatment processes allows for more efficient and optimized operations. AI-driven systems can monitor water quality in real-time, predict system failures, optimize chemical dosing, and reduce energy consumption. By leveraging AI technologies, water treatment facilities can enhance their overall performance and streamline resource utilization.
-
Decarbonation and Degasification Systems: Decarbonation and degasification are essential processes in industrial water treatment, particularly in pH levels in water and the ability to control removing the contaminants. These processes target the removal of carbon dioxide (CO2) and other dissolved gases from water to improve its quality. Two key systems used for this purpose are the decarbonator and aeration system.
Read More
Topics:
degasification,
advanced treatment solutions,
biological scrubber,
NSF/ANSI 61,
Chemical Odor,
Decarbonation,
Safe drinking water,
De-Aeration,
decarbonator,
degasifier,
degassed water,
ansi61,
nsf/ansi61,
Deagasification,
decarbonation of water,
DeLoach Industries, Inc.,
Drinking Water,
Industrial Odor Control,
DeLoach Industries,
contaminants,
process system,
safe drinking water act,
drinking water standards,
environmental safety,
air emissions,
Forced Draft,
Induced Draft
Degasification and decarbonation are essential processes in water treatment that play a crucial role in improving water quality.
Read More
Topics:
degasification,
hydrogen sulfide (H2S),
Decarbonation,
dissolved gases,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
co2 dissolved in water,
degassed water,
decarbonation of water,
DeLoach Industries, Inc.,
hydrogen sulfide molar mass,
DeLoach Industries,
carbon filters,
removing hydrogen sulfide in water,
hydrogen sulfide gas,
dissolved oxygen
Requires an application commonly referred to as either “Degasification” or "Decarbonation" and it requires the use of a piece of water treatment equipment called either a “degasifier” or a “decarbonator”.
Both of these are similar in nature and are designed for Carbon Dioxide (CO2) removal from the incoming water. A properly designed decarbonator can remove 99.99% of the free carbon dioxide gas that is present in the water stream. One of the primary reasons for utilizing a decarbonator or degasifier for the removal of carbon dioxide gas is the raise the pH of the water without the need to add caustic. resulting in high-purity water.
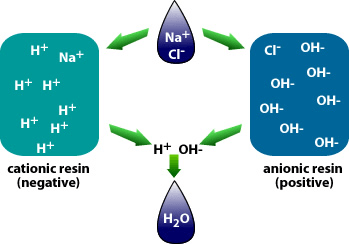
The other reason is the remove the CO2 prior to treating the water with Ion Exchange which utilizes Anion or Cation resins to reduce the regeneration cycles for the resin beds. High concentrations of CO2 consume the ion charge within the resins and require more frequent regeneration cycles. The difference between anion and cation resins is that one is positively charged (anion) and the other is negatively charged (cation), cation resins, attract positive ions with their negative charge.
The term decarbonation describes the process of the removal of suspended gas or the conversion of carbonic acids into free Carbon Dioxide. Carbonic Acid (H2CO3) is stable at normal ambient anhydrous conditions. However, Carbonic Acid decomposes when not stable and in the presence of any water molecules to form carbon dioxide (CO2). The Carbonic acid breaks down when present in water and it is converted to a gas based upon certain conditions. It is common to have CO2 present in water requiring a decarbonation process when utilizing certain types of water filtration such as membrane filtration with reverse osmosis or it can be present when the need to adjust pH is required. When removing (CO2) the process is often referred to as “Decarbonation”, when removing (H2S) Hydrogen Sulfide the process is often referred to as “Degasification”.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier
The Basics of Water Decarbonation
and the removal of carbon dioxide (CO2). The need to remove (CO2) is essential in most Aquaculture, Municipal, Industrial, and Food & Beverage Processes To understand you must familiarize yourself with Henry’s Law.
Henry's Law defines the method and proportional relationship between the amount of a gas in a solution in relation to the gas's partial pressure in the atmosphere. Often you will see and hear various terms like degasification, decarbonation, aeration, and even air stripping when discussing the removal of dissolved gases and other convertible elements from water. Understanding the impacts that Carbon Dioxide (CO2) can have on both equipment and aquatic life provides the basic reasons why the need to decarbonate water, exists. Carbon Dioxide (CO2) can exist naturally in the raw water supply or be the result of ph control and balance. In either case, the process called Decarbonation or Degasification provides the most cost-effective and efficient manner to reduce or tally remove (CO2) from the water. In addition to Carbon Dioxide (CO2), water can contain a variety of other contaminants that may impact the removal efficiency of the Carbon Dioxide. A variety of elements as well as dissolved gases such as oxygen, nitrogen, and carbon dioxide (CO2). A full analytical review of the water chemistry is required to properly design and size the “Water Treatment” process.
Breaking the bonds in water releases a dissolved gas
such as carbon dioxide (CO2) you must change the conditions of the vapor pressure surrounding the gas and allow the gas to be removed. There are many variables to consider when designing or calculating the “means and methods” of the removal of carbon dioxide (CO2). When I refer to the means and methods. I am referring to the design of a decarbonator and its components. The means equals the size and type (Hydraulic load) of the decarbonator and the “method” equals the additional variables such as the cubic foot of airflow (CFM) and “Ratio” of the air to water to accomplish the proportional condition needed to remove the carbon dioxide (CO2).
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
removal of CO2 from water
Water demineralization is also called deionization and is a process known as “Ion Exchange.”
In simple terms, water demineralization is “Water Purification.” The process involves removing dissolved ionic mineral solids from a feed-water process, typically for “Industrial” water applications. Still, it can also be utilized to remove dissolved solids from a water process for “Aquaculture,” “Food and Beverage,” and the “Municipal” markets.
Why is demineralization utilized? It can remove dissolved solids to near distilled water quality at a much lower capital and operational cost than other treatment processes such as membrane softening (Reverse Osmosis). Demineralization applies the science known as “Ion Exchange,” which attracts negative and positive charged ions and allows either to attach themselves to a negative ion depending on their respective current negative or positive charge during what is known as a resin cycle. In other technical articles, we will explore and go into more specific details on the science of the ion exchange process. Water that has dissolved salts and minerals has ions, either negatively charged ions known as “Anions” or positively charged ions known as “Cations.” To treat the water and remove these contaminants, the ions in the water are attracted to counter-ions, which have a negative charge. In a demineralization treatment process, there are pressure vessels that hold resin beads which are typically made of plastic. The beads are made from a plastic material with an ionic functional group that allows them to hold and maintain an electrostatic electrical charge. Some of these resin groups are negatively charged, referred to as “Anion” resins, while others hold a positive charge and are called “Cations” resins.
There are different applications to apply Ion exchange technologies, which is why you will often hear different terminology interchanged like deionization and demineralization. The raw water quality and the specific application will dictate the type of ion exchange process needed. For example, if the water contains a high level of hardness, the water will most likely contain Ca2+ or Mg2+ dissolved solids possessing a positive charge. To replace these hard ions, it is typical to utilize a resin bed with a salt ion like Na+. As the water passes over the resin bead material within the pressure vessel. The hard ions are replaced with the salt ion; therefore, all the hardness within the water is removed. However, the water will now contain a higher concentration of sodium ions, and this must be considered during the evaluation and selection process of the type of resin material to utilize for the specific application. If the water application requires high purity and the removal of as many solids as possible, then the term or process selected is referred to as demineralization.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
media packing,
Decarbonation,
ION Exchange Resin,
decarbonator,
degasifier,
RO system,
H2S Degasifier,
Aquaculture,
degassed water,
Co2 ph,
removal of CO2 from water,
Deagasification,
decarbonation of water,
hydrogen ion,
particulate matter,
municipal water systems,
industrial facilities,
automated control systems,
Ion exchange,
cations,
anions
Per- and polyfluorinated substances (PFAS), known as "forever chemicals," have long been utilized in various consumer products due to their exceptional properties.
However, the challenge lies in effectively treating or eliminating PFAS once they enter the environment or water supply. This blog will focus on the technological advancements in removing PFAS and perfluorooctanoic acids (PFOAs) from water sources. By exploring different treatment methods, such as activated carbon absorption, ion exchange resins, and reverse osmosis, and simply avoiding PFOA and PFOS, we can better understand the available options for mitigating these persistent chemicals in water.
Activated Carbon Absorption
One of the earliest technologies employed for PFAS removal is activated carbon absorption. This method involves the use of specially treated carbon materials that effectively adsorb PFAS compounds from water sources. The activated carbon's large surface area and porous structure allow it to trap and retain PFAS molecules. This technology has proven effective in removing PFAS, including PFOAs, from drinking water and environmental sources. However, periodic treatment and regeneration of the activated carbon are necessary to maintain its efficacy.
Read More
Topics:
degasification,
iron oxidation,
water treatment,
advanced treatment solutions,
water plant,
ION Exchange Resin,
Safe drinking water,
wastewater,
degasifier,
RO system,
Deagasification,
PFA's,
technology,
contaminants,
reverse osmosis,
carbon filters,
activated carbon,
removing PFAS & PFOS,
pfas exposure,
health effects of pfas,
nonstick cookware,
wastewater treatment systems,
PFOS,
pfoa regulations,
drinking water standards,
water resistant clothing,
environmental safety
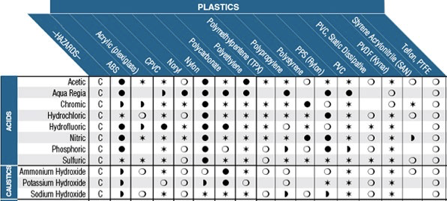
In process control systems, it is often required to handle fluids that have a harsh chemical nature. In these cases, it is necessary to be aware of material-chemical compatibility. Chemical compatibility is a general term referring to the way a specific chemical interacts with a specific material. This information is taken into consideration when selecting materials for construction for tanks, valves, pipework, tubing, and other devices that may encounter harsh chemicals. Common chemical types that are used in process systems are acids, bases, corrosives and oxidizers, and hydrocarbons. Typical chemical-resistant materials include natural and synthetic rubbers, vinyl polymers, fluoropolymers, and stainless steel. In order to determine which materials are compatible with certain chemicals, a chemical compatibility chart is often used. A chemical compatibility chart contains tabulated data about how a given material interacts with a given chemical.
Often, the manufacturer of the equipment or material in question will have their own compatibility chart for their specific goods. Most compatibility charts will have the same type of information. Materials will be categorized along one axis of the table, with fluids or gasses categorized along the other axis. At the intersection of a material with a fluid, you will find an indication of the level of compatibility. Some charts will use an A-F categorization, others may use a more graphical style. Most charts will be accompanied by a key or guide that explains how to use the table. There may also be multiple concentration levels and temperature ranges for a given fluid in cases where the distinction makes a difference with compatibility.
Read More
Topics:
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
caustic,
Decarbonation,
decarbonator,
degasifier,
Deagasification
The need to remove harmful water elements, such as Hydrogen Sulfide H2s and Carbon Dioxide CO2, from water in the pisciculture and aquaculture market is extremely important.
To achieve maximum results, the industry utilizes a treatment technology called “Degasification” and controls the pH precisely to maximize results. When utilizing equipment such as the DeLoach Industries degasification systems, the hydrogen sulfide, and carbon dioxide levels can be removed to 99.999% ug/l.
pH control with water degasification in water treatment is very important for aquaculture and the pisciculture market. In addition, there are a host of other organic and inorganic elements found in water, both naturally occurring and manmade, that require removal during some part of the water treatment process, and pH plays a significant role in the effectiveness of the treatment process.
Every application of degasification depends on pH adjustment to maximize results. As an example, the treatment of water may require the removal of hydrogen sulfide (H2S) to protect the species during the growth period. Hydrogen sulfide can be removed either as a “free” gas or requires the conversion of sulfides into (H2S) as a gas. You will often also see the need to adjust the pH of the water chemistry to maximize both the removal and the conversion to increase the efficiency of the equipment utilized to remove the hydrogen sulfide, such as a degasification tower or a degasifier.
Why is pH important, and what it means in water?
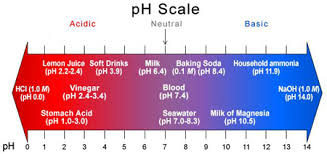
Water pH is a term used to describe whether or not the water is “acidic” or “basic.” pH ranges in water can be from 0-14. 0 is the most acidic, and 14 is at the far end and is the most basic, leaving “7” as the neutral state. A pH of 7 is neither acidic nor basic. So, what causes pH to be acidic? In nature, the most common cause of a low acidic pH in water is carbon dioxide (CO2) which occurs naturally when photosynthesis, decomposition, or respiration occurs in nature. The increase in CO2 causes an increase in ions, producing a lower pH in a simplified explanation.
How does pH play such a significant role in Aquaculture and Pisciculture?
Removing certain harmful elements is typically required to safeguard the growth of most aquatic species, and elements such as sulfides, sulfates, and free H2S hydrogen sulfide gases are dangerous. They can often kill many types of aquatic life. To maximize the removal of hydrogen sulfide from water utilizing a DeLoach Industries degasification tower, it is important to maintain as close to a pH of 5 as possible. When the pH rises above 5, the ability to convert and strip the free H2S gas from the water diminishes. When a degasification tower operates within this specific range and if it has been designed with the higher efficient distribution systems such as the ones utilized by DeLoach Industries, removal efficiencies of 99.999%- 100% can be achieved. If the pH rises to 7 or above, the removal process becomes much more difficult, and typically you will have much lower results. The pH adjustment during the water treatment process is normally accomplished by adding commercially available acid, such as “Sulphuric Acid,” one of the most common within the municipal and food and beverage industry.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
The need for pH control with water degasification and decarbonation in water treatment includes almost every industry and includes;
The need for pH control with water degasification and decarbonation in water treatment includes almost every industry and including; Aquaculture, food, and beverage, industrial, municipal, and even pisciculture. In some water treatment applications, harmful gases such as Hydrogen Sulfide (H2S) are removed, while in other applications, Carbon Dioxide (CO2) or a combination of both. In addition, there's a host of other organic and inorganic elements found in water, both naturally occurring and manmade, that require removal during some part of the water treatment process.
In almost every application of degasification or decarbonation, you will hear or see the term pH used either by need or by the result. If, as an example, the water treatment application requires the removal of Hydrogen Sulfide (H2S) to be removed either as “free” gas or requires the conversion of Sulfides into (H2S) gas. You will often also see the need to adjust the pH of the water chemistry to maximize both the removal and the conversion to increase the efficiency of the equipment being utilized to remove the hydrogen sulfide, such as a degasification tower or commonly called a degasifier.
So, what is pH?
Water pH is a term used to describe whether or not the water is “acidic” or “basic.” pH ranges in water can be from 0-14. 0 is the most acidic, and 14 is at the far end and is the most basic, leaving “7” as the neutral state. A pH of 7 is neither acidic nor basic. So, what causes pH to be acidic? In nature, the most common cause of a low acidic pH in water is Carbon Dioxide (CO2) which occurs naturally when photosynthesis, decomposition, or respiration occurs in nature. The increase in CO2 causes an increase in ions, producing a lower pH in a simplified explanation.
How does pH play such a significant role in degasification and decarbonation?
As mentioned above in the example of the removal of certain harmful elements such as sulfides, sulfates, and free H2S hydrogen sulfide gases, to maximize the removal from water utilizing a degasification tower, it is essential to maintain as close to a pH of 5 as possible. When the pH rises above 5, the ability to convert and strip the free H2S gas from the water diminishes. When a degasification tower operates within this specific range and if it has been designed with the higher efficient distribution systems such as the ones utilized by DeLoach Industries, removal efficiencies of 99.999%- 100% can be achieved. If the pH rises to seven or above, the removal process becomes much more complex, and typically, you will have much lower results. The pH adjustment during the water treatment process is typically accomplished by adding commercially available acid, such as “sulphuric acid,” one of the most common in the municipal and food and beverage industry.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
odor control,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
Chemical Odor,
pH levels,
Decarbonation,
dissolved gases,
carbon dioxide,
degasifier,
gases,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture