To enhance and control the production and quality of seafood grown and harvested.
The industry increasingly focuses on constructing in-house aquaculture fish farms, commonly called aqua farming. The most popular species of aqua farming continue to be salmon, tilapia, catfish, and carp. Increased interest in the United States has developed aqua farming facilities in southern Florida with favorable climate and water conditions.
When considering several types of fish species to grow for harvest, it is important to remember the need to control the water quality. If the aqua farm is intended to utilize man-made tanks, they will depend upon a constant flow of incoming water. If the aqua farm focuses on salmon, the water quality and temperature play a major role in the operation's mortality rates and production yields.
Having water with too high of hydrogen sulfide, carbon dioxide, total Organic carbons, and even turbidity can increase mortality rates among the younger fish species and is especially critical to salmon.
Having high levels of metals
Such as Iron that is identified as either “ferric” (Fe-) or “ferrous” (FE+2) and is naturally occurring within the Florida waters and other parts of the US will cause significant damage to young salmon species because the metal accumulates within the gills of the fish causing suffocation. Other metals are also detrimental to fish, including copper, aluminum, arsenic, cadmium, chromium, Lead, manganese, and mercury, to name a few, and the water quality must be evaluated and tested in the early stages of design to anticipate the required types of process systems needed.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
Decarbonation,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
carbonic acid,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
Water Treatment
When planning and designing a man made on land aquaculture or pisciculture facility.
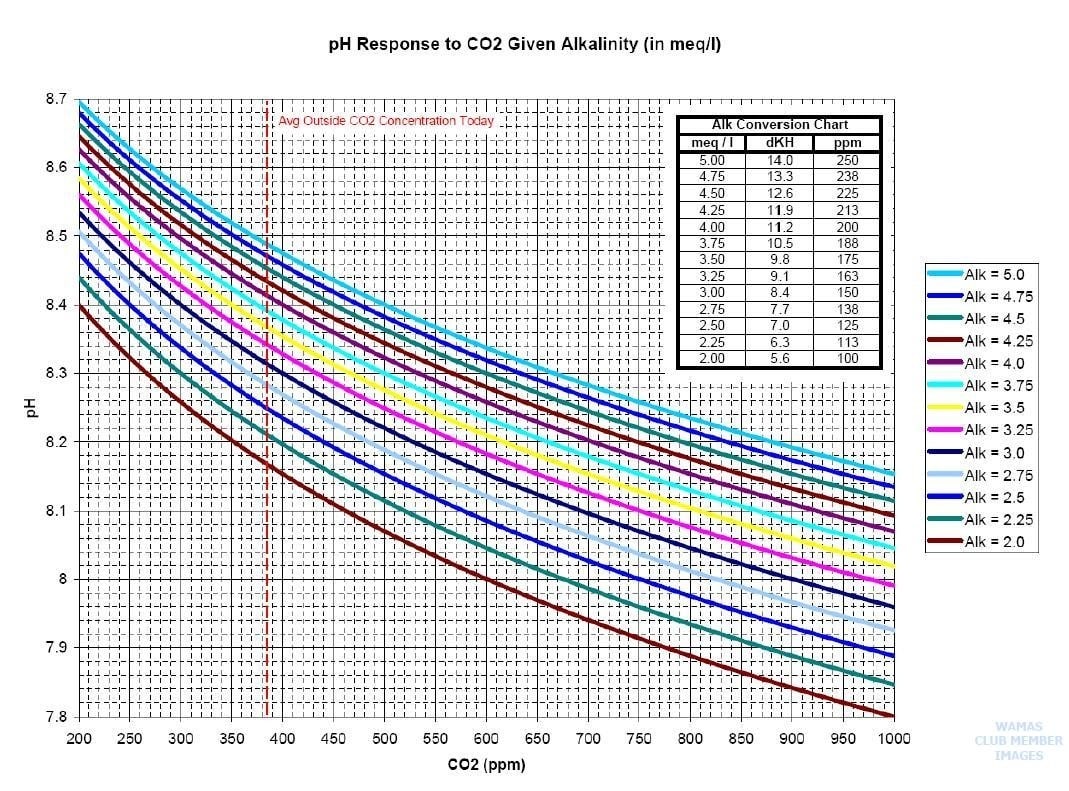
The most important key element is the quality of the water. For operations developing in Florida or the Caribbean it is important to remember that water quality varies in Florida and other states in the US and typically requires some type of water treatment. For fresh and salt water land based farms that utilize tanks located inside of a building the water needs to be treated and pure from any naturally occurring contaminants such as hydrogen sulfide (H2S), iron (Fe+), and even carbon dioxide (CO2).
The most cost effective way to treat incoming water for aquaculture farming and remove hydrogen sulfide, iron, and lower carbon dioxide is the use of a “degasification” tower. A degasification tower or degasifier is a piece of process equipment. Degasifiers can also be referred to as a “decarbonator” or “air stripper” or even “aeration tower”. The degasification tower is a vertical column designed to remove certain types of contaminants by “stripping” the molecules of converted gases and expelling them from the water as a gas. The science is based upon “Henry’s Law” and it relies upon the disproportionate varying vapor pressures of gases.
If the incoming raw water contains levels of sulfides or hydrogen sulfide gases it is recommended to remove the hydrogen sulfide to improve the water quality and reduce the risk of the development and formation of bacteria that can thrive on the Sulfur. In addition hydrogen sulfide is corrosive and will cause harm to other components within the process if left untreated. It is important to adjust the pH of the raw feed water prior to degasification to ensure full conversion of the sulfides into hydrogen sulfide gas (H2S) to enable the degasification process to perform and remove up to 99.99% of the harmful contaminants without adding additional chemicals. This saves money and improves quality of the product!
Read More
Topics:
water quality,
degasification,
pH levels of water,
water treatment,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
Decarbonation,
Caribbean,
carbon dioxide,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
Treating Hydrogen Sulfide for Environmental Safety
Read More
Topics:
odor control,
aeration,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
pH levels,
Decarbonation,
dissolved gases,
wastewater,
carbon dioxide,
degasifier,
gases,
Amine,
H2S Degasifier
DeLoach Industries made history in 1977 at the City of Cape Coral Florida water treatment plant with its large scale “degasification towers” connected to what was to become the first municipal water treatment facility in the United States to deploy the use of reverse osmosis on a large-scale production municipal treatment plant.
The Cape Coral water treatment plant for came online in 1977 and produced 3 million gallons of water per day (GPD) or 11.35 liters of purified and treated water utilizing the “reverse osmosis” process. By 1985 the plant had expanded as it kept up with growth to produce 15 million gallons per day making it at the time the world’s largest “reverse osmosis” water treatment plant facility.
Read More
Topics:
water quality,
pH levels of water,
water treatment,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
scaling,
chlorine,
caustic,
Decarbonation,
wastewater,
carbon dioxide,
degasifier,
RO membrane,
RO system,
H2S Degasifier
Caustic solution for Sodium hydroxide water treatment of Sodium Hydroxide
There are many industries that require the use of a caustic scrubber which is considered a chemical scrubber and they range from the municipal industry, mining, semiconductor markets, pulp and paper, and chemical refining. There is a wide variety of industrial processes that generate noxious or corrosive off gases that require treatment and a comparison is made between biological Vs. chemical. Often biological scrubbers have limitations due to concentrations, composition, or temperature of the contaminants and if the gas stream contains acid fumes then a biological scrubber is quickly ruled out.
The odor control selection is often fraught with choices of capital cost over operational cost and quite often comes down to familiarity from the designer or purchaser. It is always a good idea to freshen up the industrial odor control the do’s and don’t’s before selecting the final solution. If the off-gas source that needs to be treated is hydrogen sulfide (H2S) or some other type of gas stream produced by an acid or ammonia it will often require neutralization for human health reasons and to protect equipment or may be required to meet regulatory compliance. Caustic scrubbers may be either vertical or horizontal by design, but both utilize a packed media bed of either random packing or trays to allow the gas fumes to meet the recirculating caustic solution which then forces the reaction to occur.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
pH levels,
caustic,
wastewater,
carbon dioxide,
degasifier,
gases,
caustic solution,
sodium hydroxide water treatment
A Biological Scrubber is a wet odor control scrubber that treats and removes contaminants from an air stream.
It utilizes caustic typically to control the pH of the re-circulation solution. There are several types of odor control and chemical fume scrubbers on the market today. Each plays a role in treating noxious or corrosive gases in the industry.
Biological scrubbers are used in municipal applications to treat low and high hydrogen sulfide (H2S) gas levels. This colorless gas is removed from the water or wastewater treatment process.
Water treatment equipment such as “degasification” or “decarbonation” towers.
Strips the hydrogen sulfide gas from the treated wastewater and exhausts the gas from an exhaust port. These gases are captured and sent to the biological scrubber via an air duct system. The health effects of hydrogen sulfide can cause eye irritation, loss of appetite, and fluid in the lungs. Hydrogen gases are captured at a wastewater treatment process, including treatment facilities, lift stations, or head-works facilities. The PVC or FRP duct system sends the gases to the biological scrubber.
How does a Biological Scrubber work?
A biological scrubber utilizes tiny microorganisms (bacteria) to break down and digest contaminants. The bacteria feed on the contaminants and utilize this as a feed source to live and grow. When utilizing a biological scrubber for hydrogen sulfide (H2S) treatment, the by-product waste is acid from the digested H2S. This lowers the pH and requires the use of caustic to buffer the water and nutrient solution that is recirculated within the scrubber to maintain a neutral pH. The captured gas containing contaminants enters the bottom of a vertical biological scrubber. Similar to how the gas enters any other type of chemical scrubber or single or dual pass odor control scrubber.
The gas stream travels upward. Passes over a media bed that has been cultured to grow live microorganisms. A biological odor control scrubber already has “artificial intelligence” because of the millions of microbes colonies it supports.
Read More
Topics:
water treatment issues,
odor control,
advanced treatment solutions,
biological scrubber,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
dissolved gases,
wastewater,
carbon dioxide,
degasifier,
gases,
RO system,
H2S Degasifier,
what is a scrubber
Odor control and acid scrubbers are both popular in many industries.
Read More
Topics:
odor control,
water treatment,
biological scrubber,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
dissolved gases,
carbon dioxide,
degasifier,
gases,
H2S Degasifier
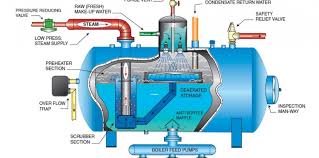
In the United States manufacturing industry, an astonishing 400 million gallons of water per day (MGD) is consumed to generate steam.
Out of this amount, approximately 60 MGD is sent to blow-down drains, while another 300 MGD is used for direct injection of steam. The common denominator in all of these processes is the need for purified and treated water. Without proper treatment, manufacturers would face frequent shutdowns and increased capital expenditure, significantly impacting their cost of goods. One effective method of water treatment to protect boilers is through degasification and deaeration.
Degasification towers play a crucial role in removing harmful gases such as hydrogen sulfide (H2S), carbon dioxide (CO2), and often dissolved oxygen (DO). The elimination of these corrosive gases is vital for enhancing the lifespan and efficiency of boiler systems. If these gases are allowed to remain in the boiler feed water, particularly carbon dioxide (CO2), it can lead to disastrous consequences, including higher operating costs and reduced system longevity. Carbon dioxide (CO2) can convert into carbonic acid, creating a corrosive environment for the boiler and other critical components. In cases where an ion exchange process is implemented prior to the boiler, the presence of carbon dioxide (CO2) can drastically increase regeneration costs as the resins are consumed. By removing carbon dioxide (CO2), the life of the resin is extended, and the pH of the water is elevated, reducing the need for additional chemicals and further lowering operating costs.
Read More
Topics:
water treatment issues,
degasification,
iron oxidation,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
Decarbonation,
ION Exchange Resin,
feed water,
De-Aeration,
steam generation,
steam generating boilers,
carbon dioxide,
steam,
decarbonator,
boiler system,
degasifier,
gases,
RO membrane,
carbonic acid,
RO system,
H2S Degasifier,
Boiler feed water
Avoid problems with calcium chlorite and corrosive gasses with your odor control scrubber.
When planning or designing an odor control system, one should pay close attention to several key variables that can cause havoc on a chemical odor control scrubber when trying to treat hydrogen sulfide or ammonia gases. The need for odor control occurs in many different forms. It is essential to understand the process that is creating the odorous or corrosive gas and the need for odor control & air emissions treatment.
First, begin to identify
all the potential obstacles that may creep up later after the chemical odor or corrosive gas control system goes online, like acid or caustic consumption. For example, chemical odor control systems designed for water treatment for the municipal industry are typically needed and attached to a degasification or decarbonation process, often needed to treat hydrogen sulfide (H2S). However, designers often may not pay close enough attention to the type of water process available for “make-up” water for the chemical scrubber. The addition of caustic can create scaling or fouling. This unknown variable of the makeup water quality can lead to a complete tower shutdown if the chemical scrubber distribution and media bed scales or fouls. The most commonly used chemicals for a hydrogen sulfide (H2S) scrubber are either chlorine in the form of sodium hypochlorite or caustic in the form of caustic soda. Both of these chemicals are common to a water treatment facility and are already in place to adjust and control pH.
The makeup water plays a significant role in the operation of a chemical scrubber.
When water containing high hardness levels is used as the source for the makeup water, your chemical scrubber can become fouled, and scaling can occur in a matter of hours, depending on the alkalinity and salts within the water. Solidification can occur from the scaling when combining sodium hypochlorite and raw feed water at specific pH ranges and these ranges are usually the range needed to achieve peak performance. Calcium chloride will form, and your chemical odor control scrubber will become a solid chunk of calcium chlorite making, making the ability for water or air to pass freely through the media packing next to impossible. No matter what type of media packing is utilized in the odor control or gas scrubber, it can foul and scale if the water chemistry is incorrect. Trust me when I say “been there and done that”! I have seen operators who have allowed a chemical scrubber to become out of balance with pH control and completely solidify the tower column to the degree that neither air nor water passage is possible. The problem can still occur with ammonia scrubbers but are different with different sets of parameters.
Read More
Topics:
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
calcium carbonate,
media packing,
pH levels,
Alkalinity,
Langilier index (LSI),
scaling,
chlorine,
caustic,
ION Exchange Resin,
Safe drinking water,
dissolved gases,
De-Aeration,
carbon dioxide,
oxygen,
degasifier,
gases,
H2S Degasifier,
calcium chlorite
The water treatment industry has developed and evolved over the years to continue to find new ways to produce degassed water,
Read More
Topics:
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
water plant,
safety,
hydrogen sulfide (H2S),
Chemical Odor,
media packing,
pH levels,
Decarbonation,
dissolved gases,
wastewater,
Global,
carbon dioxide,
decarbonator,
degasifier,
gases,
RO membrane,
H2S Degasifier,
degassed water