Degasification and decarbonation are essential processes in water treatment that play a crucial role in improving water quality.
Read More
Topics:
degasification,
hydrogen sulfide (H2S),
Decarbonation,
dissolved gases,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
co2 dissolved in water,
degassed water,
decarbonation of water,
DeLoach Industries, Inc.,
hydrogen sulfide molar mass,
DeLoach Industries,
carbon filters,
removing hydrogen sulfide in water,
hydrogen sulfide gas,
dissolved oxygen
Requires an application commonly referred to as either “Degasification” or "Decarbonation" and it requires the use of a piece of water treatment equipment called either a “degasifier” or a “decarbonator”.
Both of these are similar in nature and are designed for Carbon Dioxide (CO2) removal from the incoming water. A properly designed decarbonator can remove 99.99% of the free carbon dioxide gas that is present in the water stream. One of the primary reasons for utilizing a decarbonator or degasifier for the removal of carbon dioxide gas is the raise the pH of the water without the need to add caustic. resulting in high-purity water.
The other reason is the remove the CO2 prior to treating the water with Ion Exchange which utilizes Anion or Cation resins to reduce the regeneration cycles for the resin beds. High concentrations of CO2 consume the ion charge within the resins and require more frequent regeneration cycles. The difference between anion and cation resins is that one is positively charged (anion) and the other is negatively charged (cation), cation resins, attract positive ions with their negative charge.
The term decarbonation describes the process of the removal of suspended gas or the conversion of carbonic acids into free Carbon Dioxide. Carbonic Acid (H2CO3) is stable at normal ambient anhydrous conditions. However, Carbonic Acid decomposes when not stable and in the presence of any water molecules to form carbon dioxide (CO2). The Carbonic acid breaks down when present in water and it is converted to a gas based upon certain conditions. It is common to have CO2 present in water requiring a decarbonation process when utilizing certain types of water filtration such as membrane filtration with reverse osmosis or it can be present when the need to adjust pH is required. When removing (CO2) the process is often referred to as “Decarbonation”, when removing (H2S) Hydrogen Sulfide the process is often referred to as “Degasification”.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier
The Basics of Water Decarbonation
and the removal of carbon dioxide (CO2). The need to remove (CO2) is essential in most Aquaculture, Municipal, Industrial, and Food & Beverage Processes To understand you must familiarize yourself with Henry’s Law.
Henry's Law defines the method and proportional relationship between the amount of a gas in a solution in relation to the gas's partial pressure in the atmosphere. Often you will see and hear various terms like degasification, decarbonation, aeration, and even air stripping when discussing the removal of dissolved gases and other convertible elements from water. Understanding the impacts that Carbon Dioxide (CO2) can have on both equipment and aquatic life provides the basic reasons why the need to decarbonate water, exists. Carbon Dioxide (CO2) can exist naturally in the raw water supply or be the result of ph control and balance. In either case, the process called Decarbonation or Degasification provides the most cost-effective and efficient manner to reduce or tally remove (CO2) from the water. In addition to Carbon Dioxide (CO2), water can contain a variety of other contaminants that may impact the removal efficiency of the Carbon Dioxide. A variety of elements as well as dissolved gases such as oxygen, nitrogen, and carbon dioxide (CO2). A full analytical review of the water chemistry is required to properly design and size the “Water Treatment” process.
Breaking the bonds in water releases a dissolved gas
such as carbon dioxide (CO2) you must change the conditions of the vapor pressure surrounding the gas and allow the gas to be removed. There are many variables to consider when designing or calculating the “means and methods” of the removal of carbon dioxide (CO2). When I refer to the means and methods. I am referring to the design of a decarbonator and its components. The means equals the size and type (Hydraulic load) of the decarbonator and the “method” equals the additional variables such as the cubic foot of airflow (CFM) and “Ratio” of the air to water to accomplish the proportional condition needed to remove the carbon dioxide (CO2).
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
removal of CO2 from water
One of the most pressing issues that we face today is the phenomenon of red tide.
Red tide is algae bloom that can cause serious harm to marine life and humans.
In this article, I will explore what red tide is, what causes it, and its impact on Florida. Most importantly, the role of wastewater treatment in helping to prevent it.
What is Red Tide?
Red tide is a natural phenomenon that occurs when certain species of algae grow out of control. These algae produce toxins that harm marine life and humans. The term "red tide" comes from the reddish-brown color that the water takes on when the algae bloom. The bloom can happen in any part of the world in warm, coastal waters.
What Causes Red Tide?
Various factors cause these harmful algae to bloom.
- Changes in water temperature
- Nutrient pollution
- Ocean currents
The most common cause is nutrient pollution. Nutrient pollution occurs when excess nutrients like nitrogen and phosphorus enter the water. These nutrients can come from agricultural runoff, sewage from residential drain fields, inefficient wastewater treatment plants, septic tanks, and fertilizer.
Red Tide Blooms in Florida - History and Impact.
Florida has a long history of deadly algae blooms. The state experiences red tide almost every year, lasting for months.
It devastates the state's marine life, including fish, sea turtles, and dolphins. The algae produce toxins that can kill these animals. The dead fish can wash up on shore, causing beachgoers an unpleasant odor and an eyesore.
How Do These Microscopic Algae Affect Marine Life?
It affects marine life in a variety of ways. The algae's toxins can cause respiratory problems and neurological issues.
With most coastal outbreaks, the loss of marine life is significant. It produces toxins and decreases oxygen levels, another contributing factor to marine animal fatalities. Red tide disrupts the entire ecosystem and has been increasing in both frequencies of outbreaks as well as areas impacted.
How Does Red Tide Impact Humans?
Read More
Topics:
water treatment issues,
water treatment,
wastewater,
DeLoach Industries,
water process system,
red tide,
wastewater treatment plants,
red tide in florida,
wastewater treatment system,
cause of red tide,
water temperature,
marine life,
wastewater treatment infrastructure,
benefits of wastewater treatment,
water treatment standards
PFAS, or 'the forever chemicals, due to their long-lasting nature, are present in nonstick cookware, food packaging, and stain repellents and can cause health issues. Knowing the sources, making conscious decisions about products, limiting processed and packaged foods, and opting for safer alternatives are essential. You can protect yourself and your family from potential harm through these steps.
What is PFAS?
PFAS are a class of chemical substances used in various commercial and industrial applications, including nonstick cookware, stain repellents, and food packaging.
There are two main types of PFAS:
- Traditional PFAS
- Next-generation PFAS (also known as 'long-chain' PFAS).
Traditional PFAS include perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), which have been phased out in the United States due to health concerns.
Next-generation PFAS such as perfluorohexane sulfonic acid (PFHxS), perfluoroheptanoic acid (PFHpA), and perfluorononanoic acid (PFNA) have also been identified as contaminants in drinking water and other consumer products. Unfortunately, next-generation PFAS are not regulated by the United States Environmental Protection Agency (EPA).
Health effects of PFAS
PFAS exposure at low levels can cause a few health risks and medical conditions, such as weakened immunity, thyroid issues, and cancer. Research is ongoing to see if it can impact fetus/baby growth and development, but these results are not definitive. Pregnant women can pass on PFAS to their fetuses, which could hurt the infant's health. Also, children exposed to PFAS in their young years may be at a higher risk for getting ADHD in the future.
Read More
Topics:
water quality,
advanced treatment solutions,
Safe drinking water,
Global,
distillation,
DeLoach Industries, Inc.,
Drinking Water,
Clean Water,
Contaminated Water,
PFA's,
Water Test,
Water Test Kit,
DeLoach Industries,
make-up,
removing PFAS & PFOS,
pfas exposure,
health effects of pfas,
exposure to pfas,
nonstick cookware,
food packaging
When it comes to Odor Control and Air Emission scrubbers, you're either pleased with how yours works or highly frustrated with how it isn't.
While wet scrubbers are powerful devices that can reduce harmful air pollutants and exhaust gases, not all are created equal. An operator's most common problem with a scrubber is a lack of performance. The following are some main reasons why your scrubber may not work correctly.
When it comes to designing a wet scrubber, different manufacturers have different design standards. Creating a device that can handle high throughput with reduced cleaning cycles. Others will develop a scrubber concentrating on the maximum removal of pollutants.
The wet scrubber that you purchase should be based on your specific needs. If you buy a scrubber not designed for your particular application, you're setting yourself up for problems.
Some factors will determine how well your scrubber will perform, including the gas flow rate, gas pressure, the type of media used, and the design of the scrubber itself. If your wet scrubber design cannot handle your application's specific requirements, you'll experience reduced performance and increased downtime.
Read More
Topics:
the type of media,
gas flow rate,
wet scrubber,
wet scrubber design,
media type,
gas pressure,
gas flows,
pressure drops
If you’ve been reading the news lately, you know nanoparticles are not so great. In everything from cosmetics to water filters, nanoparticles have been shown to cause various health problems. But what exactly are nanoparticles, and how can you protect yourself from their harmful effects? Let’s answer these questions and more with this quick guide on removing nanoparticles from your drinking water.
Read More
Topics:
water treatment issues,
water quality,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
safety,
Safe drinking water,
Global,
distillation,
RO membrane,
RO system,
particulate matter,
filters,
municipal water systems,
residential well water systems,
DeLoach Industries, Inc.,
Drinking Water,
Clean Water,
Water Test,
Water Test Kit,
DeLoach Industries,
technology,
minerals,
temperature,
nanoparticles,
Cosmetics,
Nano,
make-up,
organ function,
contaminants,
pressure filters,
reverse osmosis,
carbon filters,
UV filters,
activated carbon
3D printing is a technology that has only recently become commercially available.
Progress in the last decade has allowed the equipment to excel tremendously.
3D print technology was started in 1987 by 3D Systems Corporation. The technology gained traction in the early 2010s. 3D printing is a type of additive manufacturing that creates three-dimensional parts. By successively adding material layer by layer until the part is complete.
To create the part, a 3D CAD model is required. The potential of 3D printing has led to a wide variety of technologies on the market. This blog will go into depth on the most prevalent types of 3D printing and their applications.
Figure: Direct side-by-side comparison of the three polymer 3D print technologies discussed in this blog.
Read More
Topics:
water treatment,
DeLoach Industries, Inc.,
3D CAD,
software,
3D,
DeLoach Industries,
3D parametric,
dimensions,
technology,
2D CAD,
parametric,
nylon,
abrasion,
printer,
sls,
Polymer,
geometries,
printing,
prototyping,
interlocking,
3D technology,
interior,
Polylactic Acid,
Acrylonitrile Butadiene Styrene,
Isotropic
Odor control in a manufacturing facility is essential.
It prevents potential health risks and discomfort caused by the spread of chemicals, vapors, and fumes. Additionally, excessive vapors can hinder the efficiency of exhaust and natural ventilation systems.
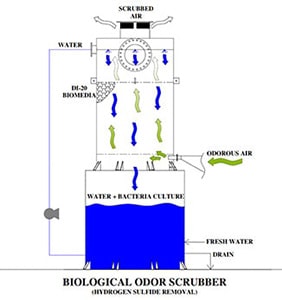
One effective solution for addressing odor issues is the installation of an Odor Control Scrubber Tower. These towers are part of the ventilation system in manufacturing plants and chemical processing facilities.
Odor control scrubbers help to remove noxious fumes and odors from exhaust and air streams. This is an effective way to improve air quality. This process involves utilizing an activated carbon filter and an ionic air filter
Key Considerations for Installing an Odor Control Scrubber Tower:
Health and Safety of Workers:
Industrial environments pose risks of exposure to hazardous fumes and gases for workers. Unhealthy odors emitted in high concentrations can jeopardize their well-being and safety. In some cases, these gases may even be combustible, adding an extra level of danger.
Odor control scrubber towers remove gases from the contaminated air, ensuring a safe working environment. These towers reduce the risk of health issues such as nausea, headaches, allergy symptoms, eye irritation, and loss of consciousness. This helps maintain worker productivity and prevents sickness caused by toxic fumes and gases.
Read More
Topics:
water treatment issues,
water quality,
odor control,
water treatment,
water distribution system,
advanced treatment solutions,
biological scrubber,
water plant,
safety,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
caustic,
Safe drinking water,
wastewater,
gases,
Biological Odor Control Scrubber,
Biological odor control,
what is a scrubber,
municipal water systems,
DeLoach Industries, Inc.,
Clean Water,
Industrial Odor Control
Water turbidity refers to how transparent or translucent the water is when examining or testing it for any use.
Water turbidity can impact food and beverage, municipal, industrial, and aquaculture operations. Turbidity is caused by suspended or dissolved particles in the water that scatter light which causes the water to appear cloudy or even murky.
Different particles can cause turbidity, including sediments such as silts and clay, fine inorganic or organic matter, algae or soluble colored organic compounds, and microscopic organisms. Turbidity is measured in a value referred to as NTU, which means Nephelometric Turbidity Unit. The EPA requires a turbidity level no higher than 0.3 NTU in the USA, and if a member of the partnership of safe drinking water, then the level must not exceed 0.1 NTU.
High turbidity can create habitats for other harmful elements, such as bacteria or metals, that can accumulate onto the particles. This increases the health risk for a potable water system. In aquaculture operations, increased turbidity from silts and sediments can harm and harm marine life, so it must be removed to safe levels. For the food and beverage industry, the impact of high turbidity can be both a safety concern and a visual and noticeable quality concern because if the turbidity is high, it can alter the physical look of the final product, for example, a distillery.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
Safe drinking water,
De-Aeration,
decarbonator,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture,
Deagasification,
particulate matter,
filters,
Sand filters,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
turbidity