Odor control in a manufacturing facility is essential.
It prevents potential health risks and discomfort caused by the spread of chemicals, vapors, and fumes. Additionally, excessive vapors can hinder the efficiency of exhaust and natural ventilation systems.
One effective solution for addressing odor issues is the installation of an Odor Control Scrubber Tower. These towers are part of the ventilation system in manufacturing plants and chemical processing facilities.
Odor control scrubbers help to remove noxious fumes and odors from exhaust and air streams. This is an effective way to improve air quality. This process involves utilizing an activated carbon filter and an ionic air filter
Key Considerations for Installing an Odor Control Scrubber Tower:
Health and Safety of Workers:
Industrial environments pose risks of exposure to hazardous fumes and gases for workers. Unhealthy odors emitted in high concentrations can jeopardize their well-being and safety. In some cases, these gases may even be combustible, adding an extra level of danger.
Odor control scrubber towers remove gases from the contaminated air, ensuring a safe working environment. These towers reduce the risk of health issues such as nausea, headaches, allergy symptoms, eye irritation, and loss of consciousness. This helps maintain worker productivity and prevents sickness caused by toxic fumes and gases.
Read More
Topics:
water treatment issues,
water quality,
odor control,
water treatment,
water distribution system,
advanced treatment solutions,
biological scrubber,
water plant,
safety,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
caustic,
Safe drinking water,
wastewater,
gases,
Biological Odor Control Scrubber,
Biological odor control,
what is a scrubber,
municipal water systems,
DeLoach Industries, Inc.,
Clean Water,
Industrial Odor Control
Water turbidity refers to how transparent or translucent the water is when examining or testing it for any use.
Water turbidity can impact food and beverage, municipal, industrial, and aquaculture operations. Turbidity is caused by suspended or dissolved particles in the water that scatter light which causes the water to appear cloudy or even murky.
Different particles can cause turbidity, including sediments such as silts and clay, fine inorganic or organic matter, algae or soluble colored organic compounds, and microscopic organisms. Turbidity is measured in a value referred to as NTU, which means Nephelometric Turbidity Unit. The EPA requires a turbidity level no higher than 0.3 NTU in the USA, and if a member of the partnership of safe drinking water, then the level must not exceed 0.1 NTU.
High turbidity can create habitats for other harmful elements, such as bacteria or metals, that can accumulate onto the particles. This increases the health risk for a potable water system. In aquaculture operations, increased turbidity from silts and sediments can harm and harm marine life, so it must be removed to safe levels. For the food and beverage industry, the impact of high turbidity can be both a safety concern and a visual and noticeable quality concern because if the turbidity is high, it can alter the physical look of the final product, for example, a distillery.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
Safe drinking water,
De-Aeration,
decarbonator,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture,
Deagasification,
particulate matter,
filters,
Sand filters,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
turbidity
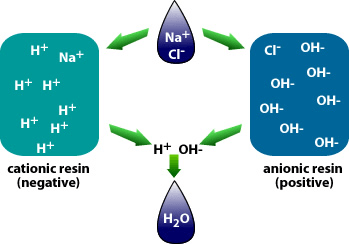
Water demineralization is also called deionization and is a process known as “Ion Exchange.”
In simple terms, water demineralization is “Water Purification.” The process involves removing dissolved ionic mineral solids from a feed-water process, typically for “Industrial” water applications. Still, it can also be utilized to remove dissolved solids from a water process for “Aquaculture,” “Food and Beverage,” and the “Municipal” markets.
Why is demineralization utilized? It can remove dissolved solids to near distilled water quality at a much lower capital and operational cost than other treatment processes such as membrane softening (Reverse Osmosis). Demineralization applies the science known as “Ion Exchange,” which attracts negative and positive charged ions and allows either to attach themselves to a negative ion depending on their respective current negative or positive charge during what is known as a resin cycle. In other technical articles, we will explore and go into more specific details on the science of the ion exchange process. Water that has dissolved salts and minerals has ions, either negatively charged ions known as “Anions” or positively charged ions known as “Cations.” To treat the water and remove these contaminants, the ions in the water are attracted to counter-ions, which have a negative charge. In a demineralization treatment process, there are pressure vessels that hold resin beads which are typically made of plastic. The beads are made from a plastic material with an ionic functional group that allows them to hold and maintain an electrostatic electrical charge. Some of these resin groups are negatively charged, referred to as “Anion” resins, while others hold a positive charge and are called “Cations” resins.
There are different applications to apply Ion exchange technologies, which is why you will often hear different terminology interchanged like deionization and demineralization. The raw water quality and the specific application will dictate the type of ion exchange process needed. For example, if the water contains a high level of hardness, the water will most likely contain Ca2+ or Mg2+ dissolved solids possessing a positive charge. To replace these hard ions, it is typical to utilize a resin bed with a salt ion like Na+. As the water passes over the resin bead material within the pressure vessel. The hard ions are replaced with the salt ion; therefore, all the hardness within the water is removed. However, the water will now contain a higher concentration of sodium ions, and this must be considered during the evaluation and selection process of the type of resin material to utilize for the specific application. If the water application requires high purity and the removal of as many solids as possible, then the term or process selected is referred to as demineralization.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
media packing,
Decarbonation,
ION Exchange Resin,
decarbonator,
degasifier,
RO system,
H2S Degasifier,
Aquaculture,
degassed water,
Co2 ph,
removal of CO2 from water,
Deagasification,
decarbonation of water,
hydrogen ion,
particulate matter,
municipal water systems,
industrial facilities,
automated control systems,
Ion exchange,
cations,
anions
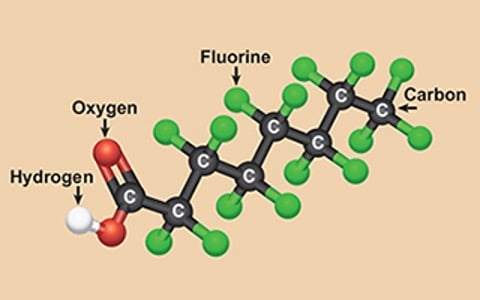
Per- and polyfluorinated substances (PFAS), known as "forever chemicals," have long been utilized in various consumer products due to their exceptional properties.
However, the challenge lies in effectively treating or eliminating PFAS once they enter the environment or water supply. This blog will focus on the technological advancements in removing PFAS and perfluorooctanoic acids (PFOAs) from water sources. By exploring different treatment methods, such as activated carbon absorption, ion exchange resins, and reverse osmosis, and simply avoiding PFOA and PFOS, we can better understand the available options for mitigating these persistent chemicals in water.
Activated Carbon Absorption
One of the earliest technologies employed for PFAS removal is activated carbon absorption. This method involves the use of specially treated carbon materials that effectively adsorb PFAS compounds from water sources. The activated carbon's large surface area and porous structure allow it to trap and retain PFAS molecules. This technology has proven effective in removing PFAS, including PFOAs, from drinking water and environmental sources. However, periodic treatment and regeneration of the activated carbon are necessary to maintain its efficacy.
Read More
Topics:
degasification,
iron oxidation,
water treatment,
advanced treatment solutions,
water plant,
ION Exchange Resin,
Safe drinking water,
wastewater,
degasifier,
RO system,
Deagasification,
PFA's,
technology,
contaminants,
reverse osmosis,
carbon filters,
activated carbon,
removing PFAS & PFOS,
pfas exposure,
health effects of pfas,
nonstick cookware,
wastewater treatment systems,
PFOS,
pfoa regulations,
drinking water standards,
water resistant clothing,
environmental safety
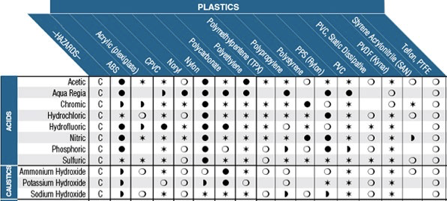
In process control systems, it is often required to handle fluids that have a harsh chemical nature. In these cases, it is necessary to be aware of material-chemical compatibility. Chemical compatibility is a general term referring to the way a specific chemical interacts with a specific material. This information is taken into consideration when selecting materials for construction for tanks, valves, pipework, tubing, and other devices that may encounter harsh chemicals. Common chemical types that are used in process systems are acids, bases, corrosives and oxidizers, and hydrocarbons. Typical chemical-resistant materials include natural and synthetic rubbers, vinyl polymers, fluoropolymers, and stainless steel. In order to determine which materials are compatible with certain chemicals, a chemical compatibility chart is often used. A chemical compatibility chart contains tabulated data about how a given material interacts with a given chemical.
Often, the manufacturer of the equipment or material in question will have their own compatibility chart for their specific goods. Most compatibility charts will have the same type of information. Materials will be categorized along one axis of the table, with fluids or gasses categorized along the other axis. At the intersection of a material with a fluid, you will find an indication of the level of compatibility. Some charts will use an A-F categorization, others may use a more graphical style. Most charts will be accompanied by a key or guide that explains how to use the table. There may also be multiple concentration levels and temperature ranges for a given fluid in cases where the distinction makes a difference with compatibility.
Read More
Topics:
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
caustic,
Decarbonation,
decarbonator,
degasifier,
Deagasification
In water treatment systems it is often important to measure the rate at which water is flowing through the system. Data from flow measurement devices can be used to control chemical dosing, set pump speeds, control filter loading rates, inform maintenance programs, and other tasks necessary for the operation of a water treatment facility or on key components such as Degasification and Decarbonation systems or Biological Odor Control Systems. As with most types of instrumentation, there is an array of technologies that can be used for the task, each one with various strengths and optimal applications. For modern electronically controlled systems, the most common types of flow sensors used are axial turbine flowmeters, paddlewheel flowmeters, differential pressure/orifice plate flow transducers, and magnetic flowmeters. This article will briefly discuss the technology and features of each of these types.
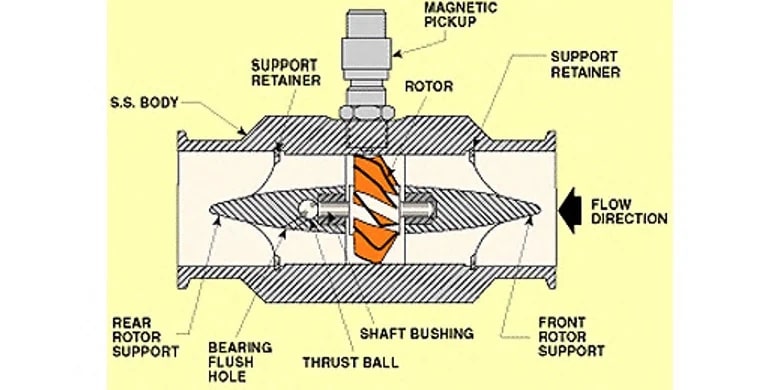
A turbine flow meter,
consists of a tube that contains supports to hold a multi-bladed metal turbine in the center. The turbine is designed to have close clearance to the walls of the tubing such that nearly all of the water is made to flow through the turbine blades as it travels through the pipe. The turbine is supported on finely finished bearings so that the turbine will spin freely even under very low flows. As the turbine spins, a magnetic pickup located outside of the flowmeter housing is used to sense the tips of the turbine blade spinning past the pickup. An amplifier/transmitter is then used to amplify the pulses and either transmit them directly or convert the pulse frequency into an analog signal that is then sent to a programmable controller for further use elsewhere in the system. One advantage of a turbine flowmeter is that the electronics are separated from the fluid path. The magnetic pickup is the only electronic component, and it is installed outside of the turbine housing, reading the presence of the turbine blade tips through the wall of the sensor body. In clean water applications, this can be advantageous because the magnetic pickup can be replaced if needed without removing the turbine from service. However, the turbine itself covers most of the pipe area and creates back pressure in the system, requiring increased pumping energy to move a given amount of water. In Industrial Water Treatment or Filtration Treatment, turbines can also easily become fouled or jammed if they are used to measure water or other fluids with entrained solids, algae or bacteria cultures which cause significant accumulation, or corrosive chemical components that can degrade the turbine bearings.
Read More
Topics:
water quality,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
pumps,
Alkalinity,
Safe drinking water,
wastewater,
Recycling,
pharmaceutical water,
Aqua Farming,
Aquaculture,
Pipe Size,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
actuated valves,
pump controls,
Drinking Water,
Clean Water,
Water Test,
Water Test Kit,
DeLoach Industries,
civil engineers
In many water treatment and chemical processes,
it is a requirement to keep track of the pH of the water or product stream. In DeLoach Industries equipment such as degasification systems, or odor control scrubbers, pH measurement is critical to control the chemical reactions happening within the treatment system. PH is an indication of the acidic vs alkaline nature of the fluid. An acidic fluid will have a greater concentration of H+ hydrogen ions, while an alkaline fluid will have a greater concentration of OH- hydroxide ions. This electrochemical nature is used in the construction, reading, and maintenance of electronic pH probes.
PH probes are generally glass and will contain a reference element, and a sensing element. When the pH probe is immersed in the fluid to be measured, the electrical potential difference between the sensing element and the reference element is amplified by electronics and the resulting voltage is used in a calculation to determine pH from differential electron potential. As a pH probe remains in service, ion exchange will slowly change the electrical potential of the sensing element, the reference element, or both. This happens because the hydrogen ions are small enough to travel through the glass sensor body and cause reference potential shifts over time. This is normal behavior for all pH probes and is the reason why pH probes must be periodically calibrated.
Calibration is a process where a pH probe is immersed in a series of standardized stable pH solutions called “buffers”. The standard set of buffers includes a pH 4.0 acidic buffer, a pH 7.0 neutral buffer, and a pH 10.0 alkaline buffer. These buffer solutions are chemically designed to hold a stable pH and are used as a reference for the internal calculations that are done by the pH amplifier or transmitter that interprets the reading taken by the pH probe. As the reference voltage vs actual pH for a mature probe changes, the known buffer solution provides a benchmark for the calculation. Each pH instrumentation manufacturer will have a slightly different method for performing a calibration, but in general, the system will have you step through the buffer solutions while an automated routine makes note of the expected voltage vs calibration voltage at each step. The computation algorithm will use this drift information to re-scale the calculation to re-establish accuracy.
Read More
Topics:
water treatment issues,
water quality,
pH levels of water,
iron oxidation,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
Alkalinity,
ION Exchange Resin,
carbon dioxide,
gases,
RO system,
Aqua Farming
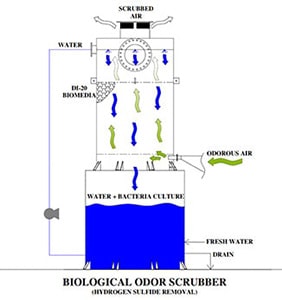
A Biological Odor Control Scrubber Is Just One Of Many Different Types Of Available Technologies to treat air emissions that may contain harmful gases.
This class of equipment commonly falls into a category referred to as “Odor Control Scrubbers” and they are utilized to remove dangerous or noxious odors from an air stream. The Biological Odor Control System has gained popularity among many end users such as municipal operators due to the reduced operating cost and more simplistic operating requirements. A typical chemical odor control scrubber often requires two or more chemical additives and more instrumentation is required to maintain system performance. With the additional chemicals required and instrumentation comes the need for more hands-on maintenance, calibration, and safety requirements which increases the operating costs and workload of the operator.
A Biological Odor Control System relies on active bacteria cultures that recirculate within a water stream and flow across a random packed media bed that is beneficial to the bacteria culture.
During the process of metabolizing harmful gases such as Hydrogen Sulfide (H2S) the biological odor control system requires only the addition of Caustic to control and balance the pH and additional water makeup to replace what has been consumed through evaporation or during the blowdown process to eliminate solids. There are several different types of odor control and chemical wet scrubbers, industrial air scrubbers on the market today and each provides a solution for the treatment of noxious or corrosive gases and odors in the industry. And even though Biological scrubbers are commonly utilized in municipal applications for the treatment of hydrogen sulfide (H2S) gases that were produced by a water or wastewater treatment process there are times when a Biological Scrubber does not provide the best solution for treatment. When there are wide or rapidly changing concentrations in the ppm (parts per million) level then a Biological Scrubber will have difficulties balancing and acclimating fast enough to prevent a breakthrough. As an example, In water treatment, there is a treatment process referred to as “degasification” which strips the hydrogen sulfide gas from the water, and then the concentrated H2S gas is exhausted from the tower through an exhaust port. When the concentration rises above 1 ppm for hydrogen sulfide gas then the levels become both noxious to the surroundings as well as corrosive. Many times, the levels range from 3-7 PPM in concentration with Hydrogen Sulfide and pose a serious health threat, noxious odor, and corrosive environment demanding capture and treatment. When utilizing an Odor Control Scrubber such as a Biological Scrubber the gases are pulled or pushed through an air duct system that is connected to the Biological Scrubber inlet or suction side of the blower. The same process is utilized when treating Hydrogen Sulfide (H2S) gases that were captured at a wastewater treatment process. These gases may have been generated from a source such as the wastewater treatment plant, lift station, or master head-works facility. When captured the gases are also conveyed in a similar manner to the Biological Odor Control Tower for treatment.
Read More
Topics:
water treatment issues,
degasification,
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
media packing,
caustic,
wastewater,
gases,
H2S Degasifier,
Ammonia,
air emissions
The need to remove harmful water elements, such as Hydrogen Sulfide H2s and Carbon Dioxide CO2, from water in the pisciculture and aquaculture market is extremely important.
To achieve maximum results, the industry utilizes a treatment technology called “Degasification” and controls the pH precisely to maximize results. When utilizing equipment such as the DeLoach Industries degasification systems, the hydrogen sulfide, and carbon dioxide levels can be removed to 99.999% ug/l.
pH control with water degasification in water treatment is very important for aquaculture and the pisciculture market. In addition, there are a host of other organic and inorganic elements found in water, both naturally occurring and manmade, that require removal during some part of the water treatment process, and pH plays a significant role in the effectiveness of the treatment process.
Every application of degasification depends on pH adjustment to maximize results. As an example, the treatment of water may require the removal of hydrogen sulfide (H2S) to protect the species during the growth period. Hydrogen sulfide can be removed either as a “free” gas or requires the conversion of sulfides into (H2S) as a gas. You will often also see the need to adjust the pH of the water chemistry to maximize both the removal and the conversion to increase the efficiency of the equipment utilized to remove the hydrogen sulfide, such as a degasification tower or a degasifier.
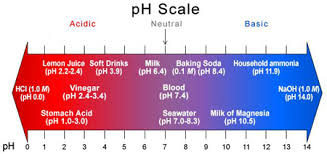
Why is pH important, and what it means in water?
Water pH is a term used to describe whether or not the water is “acidic” or “basic.” pH ranges in water can be from 0-14. 0 is the most acidic, and 14 is at the far end and is the most basic, leaving “7” as the neutral state. A pH of 7 is neither acidic nor basic. So, what causes pH to be acidic? In nature, the most common cause of a low acidic pH in water is carbon dioxide (CO2) which occurs naturally when photosynthesis, decomposition, or respiration occurs in nature. The increase in CO2 causes an increase in ions, producing a lower pH in a simplified explanation.
How does pH play such a significant role in Aquaculture and Pisciculture?
Removing certain harmful elements is typically required to safeguard the growth of most aquatic species, and elements such as sulfides, sulfates, and free H2S hydrogen sulfide gases are dangerous. They can often kill many types of aquatic life. To maximize the removal of hydrogen sulfide from water utilizing a DeLoach Industries degasification tower, it is important to maintain as close to a pH of 5 as possible. When the pH rises above 5, the ability to convert and strip the free H2S gas from the water diminishes. When a degasification tower operates within this specific range and if it has been designed with the higher efficient distribution systems such as the ones utilized by DeLoach Industries, removal efficiencies of 99.999%- 100% can be achieved. If the pH rises to 7 or above, the removal process becomes much more difficult, and typically you will have much lower results. The pH adjustment during the water treatment process is normally accomplished by adding commercially available acid, such as “Sulphuric Acid,” one of the most common within the municipal and food and beverage industry.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
The need for pH control with water degasification and decarbonation in water treatment includes almost every industry and includes;
The need for pH control with water degasification and decarbonation in water treatment includes almost every industry and including; Aquaculture, food, and beverage, industrial, municipal, and even pisciculture. In some water treatment applications, harmful gases such as Hydrogen Sulfide (H2S) are removed, while in other applications, Carbon Dioxide (CO2) or a combination of both. In addition, there's a host of other organic and inorganic elements found in water, both naturally occurring and manmade, that require removal during some part of the water treatment process.
In almost every application of degasification or decarbonation, you will hear or see the term pH used either by need or by the result. If, as an example, the water treatment application requires the removal of Hydrogen Sulfide (H2S) to be removed either as “free” gas or requires the conversion of Sulfides into (H2S) gas. You will often also see the need to adjust the pH of the water chemistry to maximize both the removal and the conversion to increase the efficiency of the equipment being utilized to remove the hydrogen sulfide, such as a degasification tower or commonly called a degasifier.
So, what is pH?
Water pH is a term used to describe whether or not the water is “acidic” or “basic.” pH ranges in water can be from 0-14. 0 is the most acidic, and 14 is at the far end and is the most basic, leaving “7” as the neutral state. A pH of 7 is neither acidic nor basic. So, what causes pH to be acidic? In nature, the most common cause of a low acidic pH in water is Carbon Dioxide (CO2) which occurs naturally when photosynthesis, decomposition, or respiration occurs in nature. The increase in CO2 causes an increase in ions, producing a lower pH in a simplified explanation.
How does pH play such a significant role in degasification and decarbonation?
As mentioned above in the example of the removal of certain harmful elements such as sulfides, sulfates, and free H2S hydrogen sulfide gases, to maximize the removal from water utilizing a degasification tower, it is essential to maintain as close to a pH of 5 as possible. When the pH rises above 5, the ability to convert and strip the free H2S gas from the water diminishes. When a degasification tower operates within this specific range and if it has been designed with the higher efficient distribution systems such as the ones utilized by DeLoach Industries, removal efficiencies of 99.999%- 100% can be achieved. If the pH rises to seven or above, the removal process becomes much more complex, and typically, you will have much lower results. The pH adjustment during the water treatment process is typically accomplished by adding commercially available acid, such as “sulphuric acid,” one of the most common in the municipal and food and beverage industry.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
odor control,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
Chemical Odor,
pH levels,
Decarbonation,
dissolved gases,
carbon dioxide,
degasifier,
gases,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture