Degasification and decarbonation are essential processes in water treatment that play a crucial role in improving water quality.
Read More
Topics:
degasification,
hydrogen sulfide (H2S),
Decarbonation,
dissolved gases,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
co2 dissolved in water,
degassed water,
decarbonation of water,
DeLoach Industries, Inc.,
hydrogen sulfide molar mass,
DeLoach Industries,
carbon filters,
removing hydrogen sulfide in water,
hydrogen sulfide gas,
dissolved oxygen
Requires an application commonly referred to as either “Degasification” or "Decarbonation" and it requires the use of a piece of water treatment equipment called either a “degasifier” or a “decarbonator”.
Both of these are similar in nature and are designed for Carbon Dioxide (CO2) removal from the incoming water. A properly designed decarbonator can remove 99.99% of the free carbon dioxide gas that is present in the water stream. One of the primary reasons for utilizing a decarbonator or degasifier for the removal of carbon dioxide gas is the raise the pH of the water without the need to add caustic. resulting in high-purity water.
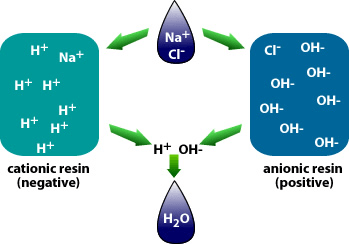
The other reason is the remove the CO2 prior to treating the water with Ion Exchange which utilizes Anion or Cation resins to reduce the regeneration cycles for the resin beds. High concentrations of CO2 consume the ion charge within the resins and require more frequent regeneration cycles. The difference between anion and cation resins is that one is positively charged (anion) and the other is negatively charged (cation), cation resins, attract positive ions with their negative charge.
The term decarbonation describes the process of the removal of suspended gas or the conversion of carbonic acids into free Carbon Dioxide. Carbonic Acid (H2CO3) is stable at normal ambient anhydrous conditions. However, Carbonic Acid decomposes when not stable and in the presence of any water molecules to form carbon dioxide (CO2). The Carbonic acid breaks down when present in water and it is converted to a gas based upon certain conditions. It is common to have CO2 present in water requiring a decarbonation process when utilizing certain types of water filtration such as membrane filtration with reverse osmosis or it can be present when the need to adjust pH is required. When removing (CO2) the process is often referred to as “Decarbonation”, when removing (H2S) Hydrogen Sulfide the process is often referred to as “Degasification”.
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
gases,
carbonic acid,
H2S Degasifier
The Basics of Water Decarbonation
and the removal of carbon dioxide (CO2). The need to remove (CO2) is essential in most Aquaculture, Municipal, Industrial, and Food & Beverage Processes To understand you must familiarize yourself with Henry’s Law.
Henry's Law defines the method and proportional relationship between the amount of a gas in a solution in relation to the gas's partial pressure in the atmosphere. Often you will see and hear various terms like degasification, decarbonation, aeration, and even air stripping when discussing the removal of dissolved gases and other convertible elements from water. Understanding the impacts that Carbon Dioxide (CO2) can have on both equipment and aquatic life provides the basic reasons why the need to decarbonate water, exists. Carbon Dioxide (CO2) can exist naturally in the raw water supply or be the result of ph control and balance. In either case, the process called Decarbonation or Degasification provides the most cost-effective and efficient manner to reduce or tally remove (CO2) from the water. In addition to Carbon Dioxide (CO2), water can contain a variety of other contaminants that may impact the removal efficiency of the Carbon Dioxide. A variety of elements as well as dissolved gases such as oxygen, nitrogen, and carbon dioxide (CO2). A full analytical review of the water chemistry is required to properly design and size the “Water Treatment” process.
Breaking the bonds in water releases a dissolved gas
such as carbon dioxide (CO2) you must change the conditions of the vapor pressure surrounding the gas and allow the gas to be removed. There are many variables to consider when designing or calculating the “means and methods” of the removal of carbon dioxide (CO2). When I refer to the means and methods. I am referring to the design of a decarbonator and its components. The means equals the size and type (Hydraulic load) of the decarbonator and the “method” equals the additional variables such as the cubic foot of airflow (CFM) and “Ratio” of the air to water to accomplish the proportional condition needed to remove the carbon dioxide (CO2).
Read More
Topics:
water treatment issues,
degasification,
pH levels of water,
aeration,
iron oxidation,
water treatment,
water plant,
bicarbonate,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
ION Exchange Resin,
dissolved gases,
De-Aeration,
wastewater,
carbon dioxide,
oxygen,
degasifier,
gases,
carbonic acid,
H2S Degasifier,
removal of CO2 from water
Water demineralization is also called deionization and is a process known as “Ion Exchange.”
In simple terms, water demineralization is “Water Purification.” The process involves removing dissolved ionic mineral solids from a feed-water process, typically for “Industrial” water applications. Still, it can also be utilized to remove dissolved solids from a water process for “Aquaculture,” “Food and Beverage,” and the “Municipal” markets.
Why is demineralization utilized? It can remove dissolved solids to near distilled water quality at a much lower capital and operational cost than other treatment processes such as membrane softening (Reverse Osmosis). Demineralization applies the science known as “Ion Exchange,” which attracts negative and positive charged ions and allows either to attach themselves to a negative ion depending on their respective current negative or positive charge during what is known as a resin cycle. In other technical articles, we will explore and go into more specific details on the science of the ion exchange process. Water that has dissolved salts and minerals has ions, either negatively charged ions known as “Anions” or positively charged ions known as “Cations.” To treat the water and remove these contaminants, the ions in the water are attracted to counter-ions, which have a negative charge. In a demineralization treatment process, there are pressure vessels that hold resin beads which are typically made of plastic. The beads are made from a plastic material with an ionic functional group that allows them to hold and maintain an electrostatic electrical charge. Some of these resin groups are negatively charged, referred to as “Anion” resins, while others hold a positive charge and are called “Cations” resins.
There are different applications to apply Ion exchange technologies, which is why you will often hear different terminology interchanged like deionization and demineralization. The raw water quality and the specific application will dictate the type of ion exchange process needed. For example, if the water contains a high level of hardness, the water will most likely contain Ca2+ or Mg2+ dissolved solids possessing a positive charge. To replace these hard ions, it is typical to utilize a resin bed with a salt ion like Na+. As the water passes over the resin bead material within the pressure vessel. The hard ions are replaced with the salt ion; therefore, all the hardness within the water is removed. However, the water will now contain a higher concentration of sodium ions, and this must be considered during the evaluation and selection process of the type of resin material to utilize for the specific application. If the water application requires high purity and the removal of as many solids as possible, then the term or process selected is referred to as demineralization.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
hydrogen sulfide (H2S),
media packing,
Decarbonation,
ION Exchange Resin,
decarbonator,
degasifier,
RO system,
H2S Degasifier,
Aquaculture,
degassed water,
Co2 ph,
removal of CO2 from water,
Deagasification,
decarbonation of water,
hydrogen ion,
particulate matter,
municipal water systems,
industrial facilities,
automated control systems,
Ion exchange,
cations,
anions
A Biological Odor Control Scrubber Is Just One Of Many Different Types Of Available Technologies to treat air emissions that may contain harmful gases.
This class of equipment commonly falls into a category referred to as “Odor Control Scrubbers” and they are utilized to remove dangerous or noxious odors from an air stream. The Biological Odor Control System has gained popularity among many end users such as municipal operators due to the reduced operating cost and more simplistic operating requirements. A typical chemical odor control scrubber often requires two or more chemical additives and more instrumentation is required to maintain system performance. With the additional chemicals required and instrumentation comes the need for more hands-on maintenance, calibration, and safety requirements which increases the operating costs and workload of the operator.
A Biological Odor Control System relies on active bacteria cultures that recirculate within a water stream and flow across a random packed media bed that is beneficial to the bacteria culture.
During the process of metabolizing harmful gases such as Hydrogen Sulfide (H2S) the biological odor control system requires only the addition of Caustic to control and balance the pH and additional water makeup to replace what has been consumed through evaporation or during the blowdown process to eliminate solids. There are several different types of odor control and chemical wet scrubbers, industrial air scrubbers on the market today and each provides a solution for the treatment of noxious or corrosive gases and odors in the industry. And even though Biological scrubbers are commonly utilized in municipal applications for the treatment of hydrogen sulfide (H2S) gases that were produced by a water or wastewater treatment process there are times when a Biological Scrubber does not provide the best solution for treatment. When there are wide or rapidly changing concentrations in the ppm (parts per million) level then a Biological Scrubber will have difficulties balancing and acclimating fast enough to prevent a breakthrough. As an example, In water treatment, there is a treatment process referred to as “degasification” which strips the hydrogen sulfide gas from the water, and then the concentrated H2S gas is exhausted from the tower through an exhaust port. When the concentration rises above 1 ppm for hydrogen sulfide gas then the levels become both noxious to the surroundings as well as corrosive. Many times, the levels range from 3-7 PPM in concentration with Hydrogen Sulfide and pose a serious health threat, noxious odor, and corrosive environment demanding capture and treatment. When utilizing an Odor Control Scrubber such as a Biological Scrubber the gases are pulled or pushed through an air duct system that is connected to the Biological Scrubber inlet or suction side of the blower. The same process is utilized when treating Hydrogen Sulfide (H2S) gases that were captured at a wastewater treatment process. These gases may have been generated from a source such as the wastewater treatment plant, lift station, or master head-works facility. When captured the gases are also conveyed in a similar manner to the Biological Odor Control Tower for treatment.
Read More
Topics:
water treatment issues,
degasification,
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
media packing,
caustic,
wastewater,
gases,
H2S Degasifier,
Ammonia,
air emissions
The need to remove harmful water elements, such as Hydrogen Sulfide H2s and Carbon Dioxide CO2, from water in the pisciculture and aquaculture market is extremely important.
To achieve maximum results, the industry utilizes a treatment technology called “Degasification” and controls the pH precisely to maximize results. When utilizing equipment such as the DeLoach Industries degasification systems, the hydrogen sulfide, and carbon dioxide levels can be removed to 99.999% ug/l.
pH control with water degasification in water treatment is very important for aquaculture and the pisciculture market. In addition, there are a host of other organic and inorganic elements found in water, both naturally occurring and manmade, that require removal during some part of the water treatment process, and pH plays a significant role in the effectiveness of the treatment process.
Every application of degasification depends on pH adjustment to maximize results. As an example, the treatment of water may require the removal of hydrogen sulfide (H2S) to protect the species during the growth period. Hydrogen sulfide can be removed either as a “free” gas or requires the conversion of sulfides into (H2S) as a gas. You will often also see the need to adjust the pH of the water chemistry to maximize both the removal and the conversion to increase the efficiency of the equipment utilized to remove the hydrogen sulfide, such as a degasification tower or a degasifier.
Why is pH important, and what it means in water?
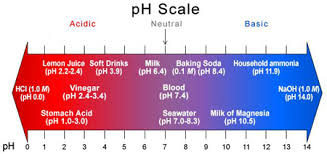
Water pH is a term used to describe whether or not the water is “acidic” or “basic.” pH ranges in water can be from 0-14. 0 is the most acidic, and 14 is at the far end and is the most basic, leaving “7” as the neutral state. A pH of 7 is neither acidic nor basic. So, what causes pH to be acidic? In nature, the most common cause of a low acidic pH in water is carbon dioxide (CO2) which occurs naturally when photosynthesis, decomposition, or respiration occurs in nature. The increase in CO2 causes an increase in ions, producing a lower pH in a simplified explanation.
How does pH play such a significant role in Aquaculture and Pisciculture?
Removing certain harmful elements is typically required to safeguard the growth of most aquatic species, and elements such as sulfides, sulfates, and free H2S hydrogen sulfide gases are dangerous. They can often kill many types of aquatic life. To maximize the removal of hydrogen sulfide from water utilizing a DeLoach Industries degasification tower, it is important to maintain as close to a pH of 5 as possible. When the pH rises above 5, the ability to convert and strip the free H2S gas from the water diminishes. When a degasification tower operates within this specific range and if it has been designed with the higher efficient distribution systems such as the ones utilized by DeLoach Industries, removal efficiencies of 99.999%- 100% can be achieved. If the pH rises to 7 or above, the removal process becomes much more difficult, and typically you will have much lower results. The pH adjustment during the water treatment process is normally accomplished by adding commercially available acid, such as “Sulphuric Acid,” one of the most common within the municipal and food and beverage industry.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
pH levels,
Decarbonation,
carbon dioxide,
oxygen,
decarbonator,
degasifier,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
The need for pH control with water degasification and decarbonation in water treatment includes almost every industry and includes;
The need for pH control with water degasification and decarbonation in water treatment includes almost every industry and including; Aquaculture, food, and beverage, industrial, municipal, and even pisciculture. In some water treatment applications, harmful gases such as Hydrogen Sulfide (H2S) are removed, while in other applications, Carbon Dioxide (CO2) or a combination of both. In addition, there's a host of other organic and inorganic elements found in water, both naturally occurring and manmade, that require removal during some part of the water treatment process.
In almost every application of degasification or decarbonation, you will hear or see the term pH used either by need or by the result. If, as an example, the water treatment application requires the removal of Hydrogen Sulfide (H2S) to be removed either as “free” gas or requires the conversion of Sulfides into (H2S) gas. You will often also see the need to adjust the pH of the water chemistry to maximize both the removal and the conversion to increase the efficiency of the equipment being utilized to remove the hydrogen sulfide, such as a degasification tower or commonly called a degasifier.
So, what is pH?
Water pH is a term used to describe whether or not the water is “acidic” or “basic.” pH ranges in water can be from 0-14. 0 is the most acidic, and 14 is at the far end and is the most basic, leaving “7” as the neutral state. A pH of 7 is neither acidic nor basic. So, what causes pH to be acidic? In nature, the most common cause of a low acidic pH in water is Carbon Dioxide (CO2) which occurs naturally when photosynthesis, decomposition, or respiration occurs in nature. The increase in CO2 causes an increase in ions, producing a lower pH in a simplified explanation.
How does pH play such a significant role in degasification and decarbonation?
As mentioned above in the example of the removal of certain harmful elements such as sulfides, sulfates, and free H2S hydrogen sulfide gases, to maximize the removal from water utilizing a degasification tower, it is essential to maintain as close to a pH of 5 as possible. When the pH rises above 5, the ability to convert and strip the free H2S gas from the water diminishes. When a degasification tower operates within this specific range and if it has been designed with the higher efficient distribution systems such as the ones utilized by DeLoach Industries, removal efficiencies of 99.999%- 100% can be achieved. If the pH rises to seven or above, the removal process becomes much more complex, and typically, you will have much lower results. The pH adjustment during the water treatment process is typically accomplished by adding commercially available acid, such as “sulphuric acid,” one of the most common in the municipal and food and beverage industry.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
odor control,
water treatment,
advanced treatment solutions,
hydrogen sulfide (H2S),
Chemical Odor,
pH levels,
Decarbonation,
dissolved gases,
carbon dioxide,
degasifier,
gases,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture
Optimizing Water Quality and Enhancing Efficiency.
To enhance and balance the water quality in aquaculture and pisciculture operations, the industry is recognizing the benefits of utilizing a type of water treatment called “Forced Draft Degasification” to remove CO2 (Carbon Dioxide) and H2S (Hydrogen Sulfide) gases and oxidize other elements such as Iron or Magnesium.
Removing these elements from the water process improves the quality of the water and aids in the balancing of the pH without the need for additional chemicals. This also reduces the risk of unnecessary and preventable loss of life to your aqua farming project. Keeping the ph at the proper level will enable a good healthy environment and will prevent further problems that may occur due to the ph is not kept in a stable state.
Read More
Topics:
De-Aeration,
carbon dioxide,
decarbonator,
degasifier,
gases,
H2S Degasifier,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture
Hydrogen Sulfide Chemical Formula and the Molar Mass of H2S.
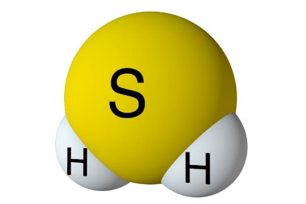
H2S is a naturally occurring chemical compound created in nature with the decay of organic material. Hydrogen sulfide is a chemical compound with a molecular formula comprised of (2) hydrogen atoms and (1) one sulfur atom. The formula is displayed as H2S. The gas is a colorless hydride, often known as the “Rotten egg gas.” This gas is very dangerous as it is poisonous and toxic to all life forms. It is also very corrosive and flammable. The H2S molar mass is 34.1 g/mol, with a melting point of -76 F (-60 C) and a melting point of –115.6F or (-82C).
Hydrogen sulfide gas is also created more often from a byproduct of a manufacturing process or the removal of water or wastewater treatment systems. In wastewater, as organic material decays, H2S is released, captured, and treated to protect human lives, reduce corrosion, H2S gas exposure, and H2S gas poisoning, H2S presence and reduce odor complaints. H2S gas is produced during manufacturing operations at refineries, pulp mills, and mining. These high levels of H2S are released during manufacturing. They must be captured and neutralized to protect human life from unwanted health effects such as pulmonary edemaand prevent excessive corrosion to your system. You cannot even smell gas at higher concentrations, and it is not distinguishable as “rotten egg gas,” which makes it even more dangerous and drives the need for hydrogen sulfide scrubbers equipment, fume scrubbers, or odor control scrubbers.
According to the “Agency for Toxic Substance & Disease Registry,” those who work within certain industries are exposed daily to higher levels of hydrogen sulfide gas than the normal public. Because the gas is also heavier than air, it will settle into lower places like manholes, tanks, and basements, and it will travel across the ground filling in low-level areas. To protect the public, OSHA (occupational safety and health administration) has set guidelines and rules known as “Permissible Exposure Limits” (PEL). A PEL is a legal limit a worker may be exposed to a chemical substance. The PEL limit for hydrogen sulfide is ten parts per million (10 ppm) over eight hours.
Read More
Topics:
water treatment issues,
degasification,
odor control,
water treatment,
advanced treatment solutions,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
dissolved gases,
wastewater,
decarbonator,
degasifier,
gases,
H2S Degasifier,
Hydrogen Sulfide Chemical Formula,
Molar mass,
Hydrogen Sulfide formula,
molar mass h2s,
hydrogen sulfide molar mass,
hydrogen sulfide gas
Basics of water decarbonation for dissolved organic carbon.
The water treatment industry continues to develop and evolve. Over the past two decades, there have been many new developments in technology and even more refinement in existing technologies such as "Degasification". The evolution and advancement of water treatment have been driven by the constantly increasing demand from an increase in population that demand cost-effective solutions and recognition to improve safety with the implementation of NSF 61 standards.
All human cultures on our planet share a single commonality: the dependency on water to survive.
Many existing technologies, such as "Degasification," have evolved with higher efficiency to meet the demand changes and provide safety to consumers and the systems. Degasification refers to the removal of dissolved gases from liquids, and the science to degasify water is based upon a chemistry equation known as "Henry's Law". The "proportionality factor" is called Henry's law constant" and was developed by William Henry in the early 19th century. Henry's Law states that "the amount of dissolved gas is proportional to its partial pressure in the gas." The most "cost" effective method to perform degasification is with the packed vertical tower called a "Degasifier” or “Decarbonator.”
The key words in this previous sentence for owners, operators, and engineers to focus on is "the most cost-effective" as there is no other process more cost-effective at removing dissolved gases at the lowest cost than using a Degasifier or decarbonator. The process of degasification is simple enough to understand. Water is pumped to the top of a vertically constructed tower, where it first enters the tower through some type of distribution system at the same time, there is a cross-current air flowing up from the bottom by a blower located at the bottom of the tower, and the air encounters the water and is exhausted at the top of the tower through an exhaust port. There are various types of distribution systems, and we will explore these in later discussions. Once the water enters the top of the tower and passes through the distribution system, it then travels by gravity downward. The next thing the water encounters is some type of media packing. There are various forms of media packing offered in the degasification industry, and each type can offer higher performance or have the ability to deter fouling. The selection of the type, size, and volume is where the “experience, engineering, and understanding of each application” comes into play.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
NSF/ANSI 61,
hydrogen sulfide (H2S),
media packing,
pH levels,
scaling,
caustic,
Decarbonation,
Safe drinking water,
dissolved gases,
carbon dioxide,
decarbonator,
boiler system,
degasifier,
carbonic acid,
H2S Degasifier,
Dissolved organic Carbon,
co2 dissolved in water