Odor control in a manufacturing facility is essential.
It prevents potential health risks and discomfort caused by the spread of chemicals, vapors, and fumes. Additionally, excessive vapors can hinder the efficiency of exhaust and natural ventilation systems.
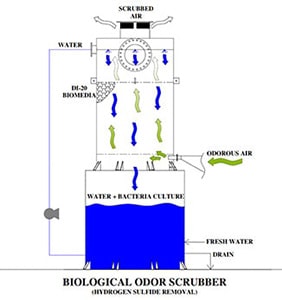
One effective solution for addressing odor issues is the installation of an Odor Control Scrubber Tower. These towers are part of the ventilation system in manufacturing plants and chemical processing facilities.
Odor control scrubbers help to remove noxious fumes and odors from exhaust and air streams. This is an effective way to improve air quality. This process involves utilizing an activated carbon filter and an ionic air filter
Key Considerations for Installing an Odor Control Scrubber Tower:
Health and Safety of Workers:
Industrial environments pose risks of exposure to hazardous fumes and gases for workers. Unhealthy odors emitted in high concentrations can jeopardize their well-being and safety. In some cases, these gases may even be combustible, adding an extra level of danger.
Odor control scrubber towers remove gases from the contaminated air, ensuring a safe working environment. These towers reduce the risk of health issues such as nausea, headaches, allergy symptoms, eye irritation, and loss of consciousness. This helps maintain worker productivity and prevents sickness caused by toxic fumes and gases.
Read More
Topics:
water treatment issues,
water quality,
odor control,
water treatment,
water distribution system,
advanced treatment solutions,
biological scrubber,
water plant,
safety,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
caustic,
Safe drinking water,
wastewater,
gases,
Biological Odor Control Scrubber,
Biological odor control,
what is a scrubber,
municipal water systems,
DeLoach Industries, Inc.,
Clean Water,
Industrial Odor Control
Water turbidity refers to how transparent or translucent the water is when examining or testing it for any use.
Water turbidity can impact food and beverage, municipal, industrial, and aquaculture operations. Turbidity is caused by suspended or dissolved particles in the water that scatter light which causes the water to appear cloudy or even murky.
Different particles can cause turbidity, including sediments such as silts and clay, fine inorganic or organic matter, algae or soluble colored organic compounds, and microscopic organisms. Turbidity is measured in a value referred to as NTU, which means Nephelometric Turbidity Unit. The EPA requires a turbidity level no higher than 0.3 NTU in the USA, and if a member of the partnership of safe drinking water, then the level must not exceed 0.1 NTU.
High turbidity can create habitats for other harmful elements, such as bacteria or metals, that can accumulate onto the particles. This increases the health risk for a potable water system. In aquaculture operations, increased turbidity from silts and sediments can harm and harm marine life, so it must be removed to safe levels. For the food and beverage industry, the impact of high turbidity can be both a safety concern and a visual and noticeable quality concern because if the turbidity is high, it can alter the physical look of the final product, for example, a distillery.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
water distribution system,
advanced treatment solutions,
water plant,
Safe drinking water,
De-Aeration,
decarbonator,
Aqua Farming,
Fish Farming,
Aquaculture,
Pisciculture,
Deagasification,
particulate matter,
filters,
Sand filters,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
turbidity
The EPA and other world health organizations have sounded the alarm on the dangers and health impacts of being exposed to per- and polyfluoroalkyl substances (PFASs & PFOAs) also known as the forever chemicals.
In response, federal and state regulators are adopting new water quality guidelines and laws to address these contaminants in our drinking water systems and groundwater pollution. It's a pervasive issue, as PFASs can be found in various types and over 4,700 different variations, each with at least three polyfluorinated carbon atoms.
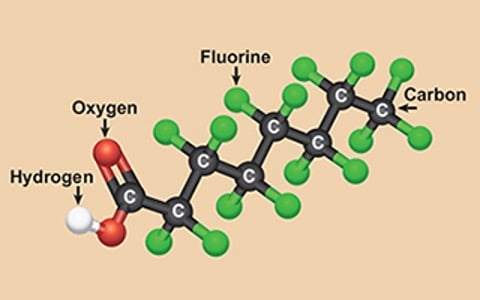
With more than 10,000 types of PFASs introduced into products, it's no wonder that the quality of drinking water in the USA and other countries has been compromised. But what exactly are PFASs? These are fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom. While they do not contain atoms like hydrogen, chlorine, bromine, or iodine, any chemical with a perfluorinated (CF3) or perfluorinated (CF2) component falls under the PFAS category. However, there are a few exceptions.
PFASs can be further classified into subgroups such as surfactants, perfluorosulfonic acids, perfluorooctane sulfonic acids, perfluorocarboxylic acids, and perfluorooctanoic acids (commonly referred to as PFOSs and PFOAs). These persistent organic pollutants, also known as "forever chemicals," pose a significant challenge due to their resistance to environmental degradation. As a result, they are found in humans, animals, and water supplies across the USA.
Read More
Topics:
degasification,
NSF/ANSI 61,
Decarbonation,
Safe drinking water,
ansi61,
Co2 ph,
CO2 in water,
Deagasification,
hydrogen ion,
DeLoach Industries, Inc.
Per- and polyfluorinated substances (PFAS), known as "forever chemicals," have long been utilized in various consumer products due to their exceptional properties.
However, the challenge lies in effectively treating or eliminating PFAS once they enter the environment or water supply. This blog will focus on the technological advancements in removing PFAS and perfluorooctanoic acids (PFOAs) from water sources. By exploring different treatment methods, such as activated carbon absorption, ion exchange resins, and reverse osmosis, and simply avoiding PFOA and PFOS, we can better understand the available options for mitigating these persistent chemicals in water.
Activated Carbon Absorption
One of the earliest technologies employed for PFAS removal is activated carbon absorption. This method involves the use of specially treated carbon materials that effectively adsorb PFAS compounds from water sources. The activated carbon's large surface area and porous structure allow it to trap and retain PFAS molecules. This technology has proven effective in removing PFAS, including PFOAs, from drinking water and environmental sources. However, periodic treatment and regeneration of the activated carbon are necessary to maintain its efficacy.
Read More
Topics:
degasification,
iron oxidation,
water treatment,
advanced treatment solutions,
water plant,
ION Exchange Resin,
Safe drinking water,
wastewater,
degasifier,
RO system,
Deagasification,
PFA's,
technology,
contaminants,
reverse osmosis,
carbon filters,
activated carbon,
removing PFAS & PFOS,
pfas exposure,
health effects of pfas,
nonstick cookware,
wastewater treatment systems,
PFOS,
pfoa regulations,
drinking water standards,
water resistant clothing,
environmental safety
In water treatment systems it is often important to measure the rate at which water is flowing through the system. Data from flow measurement devices can be used to control chemical dosing, set pump speeds, control filter loading rates, inform maintenance programs, and other tasks necessary for the operation of a water treatment facility or on key components such as Degasification and Decarbonation systems or Biological Odor Control Systems. As with most types of instrumentation, there is an array of technologies that can be used for the task, each one with various strengths and optimal applications. For modern electronically controlled systems, the most common types of flow sensors used are axial turbine flowmeters, paddlewheel flowmeters, differential pressure/orifice plate flow transducers, and magnetic flowmeters. This article will briefly discuss the technology and features of each of these types.
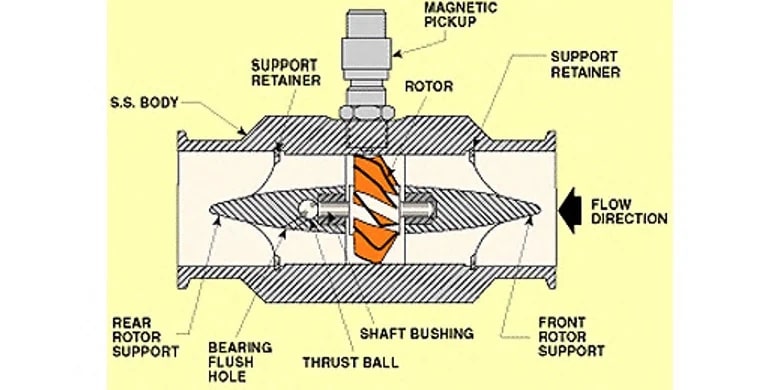
A turbine flow meter,
consists of a tube that contains supports to hold a multi-bladed metal turbine in the center. The turbine is designed to have close clearance to the walls of the tubing such that nearly all of the water is made to flow through the turbine blades as it travels through the pipe. The turbine is supported on finely finished bearings so that the turbine will spin freely even under very low flows. As the turbine spins, a magnetic pickup located outside of the flowmeter housing is used to sense the tips of the turbine blade spinning past the pickup. An amplifier/transmitter is then used to amplify the pulses and either transmit them directly or convert the pulse frequency into an analog signal that is then sent to a programmable controller for further use elsewhere in the system. One advantage of a turbine flowmeter is that the electronics are separated from the fluid path. The magnetic pickup is the only electronic component, and it is installed outside of the turbine housing, reading the presence of the turbine blade tips through the wall of the sensor body. In clean water applications, this can be advantageous because the magnetic pickup can be replaced if needed without removing the turbine from service. However, the turbine itself covers most of the pipe area and creates back pressure in the system, requiring increased pumping energy to move a given amount of water. In Industrial Water Treatment or Filtration Treatment, turbines can also easily become fouled or jammed if they are used to measure water or other fluids with entrained solids, algae or bacteria cultures which cause significant accumulation, or corrosive chemical components that can degrade the turbine bearings.
Read More
Topics:
water quality,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
pumps,
Alkalinity,
Safe drinking water,
wastewater,
Recycling,
pharmaceutical water,
Aqua Farming,
Aquaculture,
Pipe Size,
municipal water systems,
industrial facilities,
DeLoach Industries, Inc.,
actuated valves,
pump controls,
Drinking Water,
Clean Water,
Water Test,
Water Test Kit,
DeLoach Industries,
civil engineers
The process of Decarbonation requires the use of a Vertically Designed Degasification Tower.
A Decarbonation tower is specifically designed to remove Carbon Dioxide (CO2) from a water treatment process. When the water is being treated for “potable” use for direct or indirect consumption then all of the components used in the process must comply with NSF/ANSI 61 standards to assure that the components that have direct contact with the water are safe and will not introduce any foreign substance during the treatment process. Decarbonation towers have direct exposure and contact with the water during the removal of CO2 and therefore must be manufactured from a material such as Fiberglass (FRP) that complies with and meets the NSF/ANSI 61 standard.
Fiberglass FRP tanks and towers complying with NSF/ANSI 61 standards require the use of certified FRP resins and protocols during the fabrication process of the Decarbonation Tower.
It is normal protocol at DeLoach Industries Inc. to install a veil lining inside of a Decarbonator or Degasification tower that complies with NSF/ANSI 61 standards to safeguard all potable water projects. Decarbonators are utilized in the food and beverage industry, municipal industry, pisciculture, semiconductor industry, and other industrial markets. In addition to the Decarbonator complying with the NSF/ANSI 61 standard, it is equally important that the fiberglass (FRP) tanks also comply with the same standard and follow the same protocols during the manufacturing process. The NSF/ANSI 61 standard was developed to safeguard the public and provide assurances that water is free from impurities.
Read More
Topics:
water treatment,
NSF/ANSI 61,
Safe drinking water
Basics of water decarbonation for dissolved organic carbon.
The water treatment industry continues to develop and evolve. Over the past two decades, there have been many new developments in technology and even more refinement in existing technologies such as "Degasification". The evolution and advancement of water treatment have been driven by the constantly increasing demand from an increase in population that demand cost-effective solutions and recognition to improve safety with the implementation of NSF 61 standards.
All human cultures on our planet share a single commonality: the dependency on water to survive.
Many existing technologies, such as "Degasification," have evolved with higher efficiency to meet the demand changes and provide safety to consumers and the systems. Degasification refers to the removal of dissolved gases from liquids, and the science to degasify water is based upon a chemistry equation known as "Henry's Law". The "proportionality factor" is called Henry's law constant" and was developed by William Henry in the early 19th century. Henry's Law states that "the amount of dissolved gas is proportional to its partial pressure in the gas." The most "cost" effective method to perform degasification is with the packed vertical tower called a "Degasifier” or “Decarbonator.”
The key words in this previous sentence for owners, operators, and engineers to focus on is "the most cost-effective" as there is no other process more cost-effective at removing dissolved gases at the lowest cost than using a Degasifier or decarbonator. The process of degasification is simple enough to understand. Water is pumped to the top of a vertically constructed tower, where it first enters the tower through some type of distribution system at the same time, there is a cross-current air flowing up from the bottom by a blower located at the bottom of the tower, and the air encounters the water and is exhausted at the top of the tower through an exhaust port. There are various types of distribution systems, and we will explore these in later discussions. Once the water enters the top of the tower and passes through the distribution system, it then travels by gravity downward. The next thing the water encounters is some type of media packing. There are various forms of media packing offered in the degasification industry, and each type can offer higher performance or have the ability to deter fouling. The selection of the type, size, and volume is where the “experience, engineering, and understanding of each application” comes into play.
Read More
Topics:
water treatment issues,
water quality,
degasification,
pH levels of water,
water treatment,
advanced treatment solutions,
About DeLoach Industries,
water plant,
NSF/ANSI 61,
hydrogen sulfide (H2S),
media packing,
pH levels,
scaling,
caustic,
Decarbonation,
Safe drinking water,
dissolved gases,
carbon dioxide,
decarbonator,
boiler system,
degasifier,
carbonic acid,
H2S Degasifier,
Dissolved organic Carbon,
co2 dissolved in water
Avoid problems with calcium chlorite and corrosive gasses with your odor control scrubber.
When planning or designing an odor control system, one should pay close attention to several key variables that can cause havoc on a chemical odor control scrubber when trying to treat hydrogen sulfide or ammonia gases. The need for odor control occurs in many different forms. It is essential to understand the process that is creating the odorous or corrosive gas and the need for odor control & air emissions treatment.
First, begin to identify
all the potential obstacles that may creep up later after the chemical odor or corrosive gas control system goes online, like acid or caustic consumption. For example, chemical odor control systems designed for water treatment for the municipal industry are typically needed and attached to a degasification or decarbonation process, often needed to treat hydrogen sulfide (H2S). However, designers often may not pay close enough attention to the type of water process available for “make-up” water for the chemical scrubber. The addition of caustic can create scaling or fouling. This unknown variable of the makeup water quality can lead to a complete tower shutdown if the chemical scrubber distribution and media bed scales or fouls. The most commonly used chemicals for a hydrogen sulfide (H2S) scrubber are either chlorine in the form of sodium hypochlorite or caustic in the form of caustic soda. Both of these chemicals are common to a water treatment facility and are already in place to adjust and control pH.
The makeup water plays a significant role in the operation of a chemical scrubber.
When water containing high hardness levels is used as the source for the makeup water, your chemical scrubber can become fouled, and scaling can occur in a matter of hours, depending on the alkalinity and salts within the water. Solidification can occur from the scaling when combining sodium hypochlorite and raw feed water at specific pH ranges and these ranges are usually the range needed to achieve peak performance. Calcium chloride will form, and your chemical odor control scrubber will become a solid chunk of calcium chlorite making, making the ability for water or air to pass freely through the media packing next to impossible. No matter what type of media packing is utilized in the odor control or gas scrubber, it can foul and scale if the water chemistry is incorrect. Trust me when I say “been there and done that”! I have seen operators who have allowed a chemical scrubber to become out of balance with pH control and completely solidify the tower column to the degree that neither air nor water passage is possible. The problem can still occur with ammonia scrubbers but are different with different sets of parameters.
Read More
Topics:
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
odor control scrubber,
hydrogen sulfide (H2S),
calcium carbonate,
media packing,
pH levels,
Alkalinity,
Langilier index (LSI),
scaling,
chlorine,
caustic,
ION Exchange Resin,
Safe drinking water,
dissolved gases,
De-Aeration,
carbon dioxide,
oxygen,
degasifier,
gases,
H2S Degasifier,
calcium chlorite
In the past two decades, there has been a remarkable development and improvement in wastewater technologies, driven by both necessity and stringent governmental regulations.
Today, municipalities and countries worldwide are recognizing the vital importance of recycling wastewater into clean drinking water. In certain regions like the Caribbean and other foreign nations, the wastewater to the drinking water industry is not merely a choice but a necessity.
To address our global needs and challenges, the recycling of wastewater to produce safe drinking water has become an everyday practice, empowered by cutting-edge technologies such as "Ultra-Filtration" and "Membrane Bio-Reactors" (MBR). These technologies continue to advance, offering much-needed solutions to the world's water scarcity issues. Moreover, due to stricter governmental requirements for wastewater recycling, the purity standards achieved through this process often surpass those of conventional water treatment methods. To foster global growth, it is crucial for professionals and consumers alike to acknowledge and embrace wastewater recycling whenever and wherever it is applicable to meet our evolving needs.
One of the key elements in the wastewater recycling process is the removal of contaminants, such as hydrogen sulfide gas, through advanced treatment methods. Hydrogen sulfide gas, a common byproduct of various industrial processes, can pose significant risks to water quality. Through technologies like Ultra-Filtration, this harmful gas can be effectively eliminated, ensuring the production of safe drinking water.
Another crucial aspect of wastewater treatment is addressing water turbidity. Turbidity refers to the cloudiness or haziness of water caused by the presence of suspended particles. By employing techniques like Membrane Bio-Reactors (MBR), wastewater can undergo thorough filtration, effectively removing suspended solids and improving water clarity. This ensures that the recycled water meets stringent purity standards and is suitable for drinking.
Read More
Topics:
water quality,
advanced treatment solutions,
Safe drinking water,
wastewater,
Recycling,
Caribbean,
Global
Water treatment towers and storage tanks are high places that require special precautions when entering. While the majority of people who enter these locations for work can be trusted, there are some hazards that make it more important than usual to follow safety procedures.
These locations can get very hot and humid, and can also be filled with harmful chemicals and microorganisms that can cause serious health issues if inhaled or absorbed through the skin. Therefore, the general standard for workplace safety is much higher when entering locations like these.
Make sure you have read and understood the following information about safety when entering a water treatment plant. It will help you understand how to stay safe and protect yourself from harm when entering a water treatment plant. normal installation, maintenance, or even emergency repairs, it is often required to enter into a water treatment tower (degasifier, air stripper, decarbonator, or clear well/ storage tank). When this occurs, full safety protocols should be followed at all times, in accordance with OSHA regulations. A tower or tank B classification is a "Confined Space" location. For more information visit the OSHA confined space regulations page.
In addition, there are other safety risks that an operator or technician can be exposed to while inside these types of closed locations. The risk can come from fumes of hydrogen sulfide (H2S), chlorine from an injection line, or a lack of oxygen O2. A proper confined space permit should be prepared and only technicians with proper training and certifications should enter into these types of confined spaces.
Read More
Topics:
water treatment issues,
water quality,
odor control,
water treatment,
advanced treatment solutions,
biological scrubber,
water plant,
safety,
odor control scrubber,
hydrogen sulfide (H2S),
Chemical Odor,
media packing,
scaling,
caustic,
Safe drinking water,
dissolved gases,
wastewater,
carbon dioxide,
degasifier,
gases,
Ammonia,
what is a scrubber,
Hydrogen Sulfide formula,
Deagasification,
Filter Media,
DeLoach Industries, Inc.,
Drinking Water,
Clean Water,
Contaminated Water,
OSHA